|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
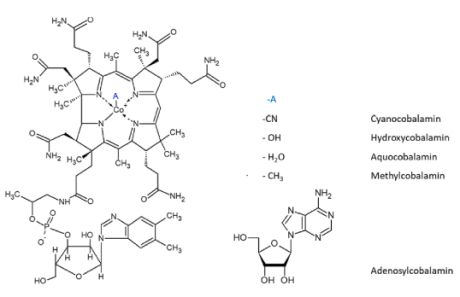
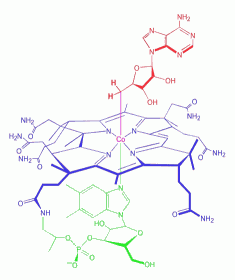 Adenosylcobalamin
is a cobalamin derivative in which the ß-substituent is deoxyadenosyl
(see the red ligand in left figure). It is one of two metabolically
active forms synthesized upon ingestion of vitamin B12 and is the
predominant form in the liver; it acts as a coenzyme in the reaction
catalyzed by methylmalonyl-CoA mutase. Adenosylcobalamin
is a cobalamin derivative in which the ß-substituent is deoxyadenosyl
(see the red ligand in left figure). It is one of two metabolically
active forms synthesized upon ingestion of vitamin B12 and is the
predominant form in the liver; it acts as a coenzyme in the reaction
catalyzed by methylmalonyl-CoA mutase.
Cobalamins are coenzymatically active forms of vitamin B12
(cyanocobalamin) which is a water-soluble vitamin and a
nutrient essential for all cells. Cobalamin is a
substituted corrin-Co(III) complex (see blue structure in left figure)
in which the cobalt atom is bound to the four nitrogen atoms of the
corrin ring, an axial group R and 5,6-dimethylbenzimidazole (DMBI, see
green part in left figure). The latter is linked to the cobalt by the
N-3 nitrogen atom and is bound to the C-1 carbon of a ribose molecule
by the N-1 nitrogen atom. Various forms of the vitamin are known with
different R groups such as R = CN, cyanocobalamin; R = OH,
hydroxocobalamin; R = CH3, methylcobalamin; R = adenosyl, coenzyme B-12
(shown here).
|
| |
|
A general term describing mass spectrometry in which ionisation takes place at ambient pressure and temperature, usually in the open air. Specific types include DESI and DART.
|
| |
|
|
the ion-exchange procedure used for the separation of anions. Both resins and bonded phases are available for this mode. The tetralkylammonium group is a typical strong anion-exchange functional group. An amino group bonded on the rigid adsorbent surface would be the example of a weak anion exchanger (WAX).
|
| |
|
|
a synthetic seawater medium specifically designed for studying metal-algae interactions
|
| |
|
Asymmetrical flow FFF (AFFFF or AF4) is a type of flow FFF using only one permeable wall which serves as accumulation wall. Experiments show that the asymmetrical design separates faster and more efficient than the symmetrical channel.
AFFFF is certainly one of the most universal FFF techniques. It features a very broad (105) separation range from 1 nm to 100 µm . And its application range covers both simple and complex macromaterials of biological, pharmaceutical, industrial and environmental relevance such as proteins, polymers or nanoparticles.
|
| |
|
Atmospheric pressure ionization (API) is any process in which ions are formed from atoms or molecules at atmospheric pressue, such as in ESI, APCI, APPI and MALDI.
|
| |
|
A form of API in which the analyte is sprayed into the source, as in ESI and APCI sources, in a solvent frequently containing a dopant molecule such as toluene or acetone. The spray is irradiated by a powerful UV source that forms excited species which undergo secondary ion/radical- molecule reactions with the analytes to cause ionisation. It is claimed to be the method of choice for detection of non-polar molecules in HPLC eluates. Protonated or deprotonated molecules or radical molecular ions can be formed, according to the mechanisms and thermodynamics involved.
|
| |
|
|
A device designed to produce a collimated beam of energetic atoms from a heated source. In MS, this is the prime source for the formation of ions in a FAB source. Most SIMS and FAB sources use atom as well as ion guns, commonly with xenon atoms or caesium ions.
|
| |
|
a hard brittle, gray-white metal. Resistant to oxidation at ordinary temperatures. Used in computer parts, x-ray tubes, gyroscopes and rocket fuel additive.
Hazard: Highly toxic, especially by inhalation of dust. Long term exposure may cause weight loss, weakness, cough, extreme difficulty in breathing and cardiac failure.
|
| |
|
Transfer of nucleic acids or proteins from an electrophoretic gel strip to a chemically reactive paper or membrane (such as nitrocellulose paper) or matrix (nylon,, for5 example) - to which they bind. Blotting is achieved through capillary diffusion (when the gel is placed between the paper or matrix and an absorptive pad) or through electrophoresis (electroblotting). Of the three types of blots, Southern hybridization (or Southern blot) transfers DNA; Northern blots transfer RNA, and Western blots transfer proteins (also called protein blots).
|
| |
|
 Bis(maltolato)oxovanadium(IV) (BMOV) can be synthesized by simple metathesis of vanadyl sulfate trihydrate and maltol (3-hydroxy-2-methyl-4-pyrone) (1:2). The ligand itself is commercially available and is an approved food additive in many countries, including Canada, the United Kingdom, and the United States. BMOV can be prepared in >90% yield in water, has a molecular weight of 317 and is soluble (millimolar scale) in a number of organic solvents as well as water. These properties together (neutral charge and aqueous solubility) contribute to high oral bioavailability. With these considerations in mind, the pentacoordinate, oxovanadium(IV) complex was developed, specifically as a potential insulin-mimetic agent. Bis(maltolato)oxovanadium(IV) (BMOV) can be synthesized by simple metathesis of vanadyl sulfate trihydrate and maltol (3-hydroxy-2-methyl-4-pyrone) (1:2). The ligand itself is commercially available and is an approved food additive in many countries, including Canada, the United Kingdom, and the United States. BMOV can be prepared in >90% yield in water, has a molecular weight of 317 and is soluble (millimolar scale) in a number of organic solvents as well as water. These properties together (neutral charge and aqueous solubility) contribute to high oral bioavailability. With these considerations in mind, the pentacoordinate, oxovanadium(IV) complex was developed, specifically as a potential insulin-mimetic agent.
|
| |
|
|
relatively coarse uncombusted or partly combusted residue of incineration that accumulates on the grate of a furnace
|
| |
|
|
A solution that maintains constant pH by resisting changes in pH from dilution or addition of small amounts of acids and bases.
|
| |
|
|
Capillary electrochromatography (CEC) is a hybrid technique in which capillary columns are packed with chromatographic sorbents and electroosmotic flow rather than pressure moves mobile phase through the column; technique has the surfacemediated selectivity potential of HPLC and the high efficiency of capillary electrophoresis (CE).
|
| |
|
|
Electrophoretic separation technique performed in a small fused silica capillary.
|
| |
|
|
The main separation mechanism in CGE is based on differences in solute size as analytes migrate through the pores of the gel-filled column. Gels are potentially useful for electrophoretic separations mainly because they permit separation based on 'molecular sieving'. They serve as anti-convective media, minimize solute diffusion, which contributes to zone broadening, prevent solute adsorption to the capillary walls and they help to eliminate electroosmosis.
|
| |
|
|
The main feature of CITP is that it is performed in a discontinuous buffer system. Sample components condense between leading and terminating constituents, producing a steady-state migrating configuration composed of conservative sample zones. This mode of operation is therefore different from other modes of capillary electrophoresis, eg CZE, which are normally carried out in a uniform carrier buffer and is characterized by sample zones which continuously change shape and relative position. In the case of a typical CZE separation, the electropherogram obtained contains sample peaks similar to those obtained in chromatographic separations, whereas in the case of CITP, the isotachopherogram obtained contains a series of steps, with each step representing an analyte zone. Unlike in other CE modes, where the amount of sample present can be determined from the area under the peaks as in chromatography, quantitation in CITP is mainly based on the measured zone length which is proportional to the amount of sample present.
|
| |
|
|
Capillary zone electrophoresis (CZE) is an electrophoretic separation technique using an electrolyte-filled capillary. When an electric field is applied across the capillary, ionic species migrate towards the oppositely charged electrode with a linear velocity depending on the electrophoretic mobility of the species in the electrolyte. Because of their solvation, the ion movement drags the bulk solution in the capillary towards the cathode resulting in an electroosmotic flow.
|
| |
|
the form of ion-exchange chromatography that uses resins or packing with functional groups that can separate cations. A sulfonic acid would be an example of a strong cation-exchange group; a carboxylic acid would be a weak cation-exchange group.
|
| |
|
|
An ion exchange resin in which the counter-ions are retained by a chelate functional group on the resin.
|
| |
|
|
A commercially available chelating resin, having a styrene-divinylbenzene copolymer incorporating iminodiacetate chelating groups.
|
| |
|
|
Chemometrics is the chemical discipline that uses mathematical and statistical methods to design or select optimal procedures and experiments, and to provide maximum chemical information by analysing chemical data.
|
| |
|
a hard, brittle, semi-gray metal. Uses includes alloys and plating element on metal and plastic substrates for corrosion resistance. Protective coating for automotive and equipment accessories.
Hazard: Hexavalent chromium compounds are suspected of producing tumors of the lungs, and nasal cavity. Corrosive action on the skin and mucous membranes.
|
| |
|
Low-molecular-weight chromium-binding substance (LMWCr; also known as chromodulin) is an oligopeptide that seems to transport chromium in the body. It consists of four amino acid residues; aspartate, cysteine, glutamate, and glycine, bonded with four (Cr3+) centers. It interacts with the insulin receptor, and has been confused with glucose tolerance factor .
Source: Wikipedia
|
| |
|
Uranium having a percentage of uranium-235 smaller than the 0.7 percent found in natural uranium. It is obtained from spent (used) fuel elements or as byproduct tails, or residues, from uranium isotope separation.
|
| |
|
Design of Experiments (DOE) is an optimal
method for planning scientific experimentation.
The use of DOE ensures maximum information return for minimum investment in time and resources. DOE selects a diverse and representative set of experiments in which all factors are independent of each other despite being varied simultaneously. The result is a causal predictive model showing the importance of all factors and their interactions. These models can be summarized as informative contour plots highlighting the optimum combination of factor settings.
|
| |
|
|
An ionisation technique that takes place outside of the mass spectrometer, at ambient temperature and pressure. A fine spray of charged droplets, formed by pneumatically assisted electrospray ionisation at high potential, typically 2-5 kV, is directed towards the sample on a surface. Ions produced from the sample are drawn into the mass spectrometer via a vacuum interface. Most surfaces and analytes are amenable and no sample preparation or pre-separation is required. Analytes may be analysed in situ, such as explosives on luggage, drugs in urine and metabolites in tissue.
|
| |
|
|
A dietary supplement is a foodstuff, administrated to supplement the normal diet, which is a concentrated source of vitamins or minerals, or other substances with a nutritional or physiological effect, marketed in a form that allows the dosage including capsules, tablets, and other similar forms, sachets of powder, ampoules of liquid, drop-dispensing bottles, and other similar forms of liquids and powders designed to be eaten in small, measured amounts.
|
| |
|
|
Transmitting or reflecting optical element with regularly spaced scribes or grooves
on its surface, designed to use phase-dependent constructive
interference to separate light by dispersing it at wavelength-dependent
angles.
|
| |
|
Any substance which when absorbed into a living organism may modify one or more of its functions.
Comment: The term is generally accepted for a substance taken for a therapeutic purpose, but is also commonly used for substances of abuse. Just as any substance can be a toxicant, so any substance can be a drug. The term carries with it the implication of use for medical purposes, but also the potential for abuse to produce an effect desired by the abuser, but which is ultimately harmful.
|
| |
|
|
A diffraction grating designed to operate with incident and diffracted
light at an angle greater than 45° from the grating normal.
|
| |
|
|
A form of molecular spectroscopy concerned with microwave-induced transitions between magnetic energy levels of electrons having a net spin and orbital angular momentum. The spectrum is normally obtained by magnetic field scanning. Also known as electron spin resonance (ESR) spectroscopy or electron magnetic resonance (EMR) spectroscopy.
The frequency (ν) of the oscillating magnetic field to induce transitions between the magnetic energy levels of electrons is measured in gigahertz (GHz) or megahertz (MHz). The following band designations are used: L (1.1 GHz), S (3.0 GHz) , X (9.5 GHz), K (22.0 GHz) and Q (35.0 GHz). The static magnetic field at which the EPR spectrometer operates is measured by the magnetic flux density (B) and its recommended unit is the tesla (T). In the absence of nuclear hyperfine interactions, B and are related by : h ν = g µBB where h is the Planck constant, µB is the Bohr magneton, and the dimensionless scalar g is called the g-factor. When the paramagnetic species exhibits an anisotropy, the spatial dependency of the g-factor is represented by a 3x3 matrix. The interaction energy between the electron spin and a magnetic nucleus is characterized by the hyperfine coupling constant A. When the paramagnetic species has anisotropy, the hyperfine coupling is expressed by a 3x3 matrix called a hyperfine coupling matrix. Hyperfine interaction usually results in splitting of lines in an EPR spectrum. The nuclear species giving rise to the hyperfine interaction should be explicitly stated, e.g."the hyperfine splitting due to 65Cu". When additional hyperfine splittings due to other nuclearspecies are resolved ("superhyperfine"), the nomenclature should include the designation of the nucleus, and the isotopic number
|
| |
|
|
Electrophoresis is based on the migration of charged molecules in solution in response to an electric field. Their rate of migration depends on the strength of the field; on the nett charge, size and shape of the molecules and also on the ionic strength, viscosity and temperature of the medium in which the molecules are moving. As an analytical tool, electrophoresis is simple, rapid and highly sensitive. It is used analytically to study the properties of a single charged species, and as a separation technique.
|
| |
|
|
- the factor that determines the rate at which a given ionic solute may move by electrophoresis.
|
| |
|
|
A mass spectrometric technique for liquid samples that involves preparing electrically charged droplets from analyte molecules dissolved in a solvent. The electrically charged droplets enter a vacuum chamber where the solvent is evaporated. Evaporation of solvent reduces the droplet size, thereby increasing the coulombic repulsion within the droplet. As the charged droplets get smaller, the excess charge within them causes them to disintegrate and release analyte molecules. The volatilized analyte molecules are then analyzed by mass spectrometry.
|
| |
|
|
In elemental mass spectrometry, a technique used mostly for inorganic
materials, the elemental composition of a sample is determined
rather
than the structural identities of its chemical constituents.
Elemental
mass spectrometry provides quantitative information about the
concentrations
of those elements. The ion source used in elemental MS is
ordinarily
an atmospheric-pressure discharge such as the inductively
coupled plasma
(ICP) or a moderate-power device such as the glow-discharge
source.
In either case, the decomposition of the sample into its
constituent
atoms and ionization of those atoms occurs in a specially
designed source.
The resulting atomic-ion beam is then separated or sorted by a
mass
spectrometer and the signal as a function of m/z used to
determine the
sample composition.
|
| |
|
|
Process whereby an enzyme is synthesized in response to the presence of a specific substance or to other agents such as heat or a metal.
|
| |
|
|
A statistical test of significance in which the
difference between two variances is tested. A variance is the square of
a standard deviation. The F-test is often used to compare the
imprecision of two analytical methods. The hypothesis being tested
(called a null hypothesis) is that there is no difference between the
two variances. If the calculated F-value is greater than the critical
value which is obtained from a statistics table, then the null
hypothesis is rejected. This means that a difference exists and that the
difference is statistically significant, or real. If the calculated
F-value is less than the critical value, the null hypothesis cannot be
rejected, therefore, there is no difference between the two variances
being tested, and the difference is not statistically significant.
|
| |
|
|
A generic name given to the housing that contains a series of optical components that focus ions onto the detector of a time-of-flight (TOF) mass analyzer. There are basically two different kinds of flight tubes that are used in commercial TOF mass analyzers. One is the orthogonal design, where the flight tube is positioned at right angles to the sampled ion beam, and the other, the axial design, where the flight tube is in the same axis as the ion beam. In both designs, all ions are sampled through the interface region, but instead of being focused into the mass filter in the conventional sequential way, packets (groups) of ions are electrostatically injected into the flight tube at exactly the same time.
|
| |
|
|
a synthetic freshwater culture medium specifically designed for studying metal-algae interactions
|
| |
|
|
Fullerene also called Buckyball or C60 is one of three known pure forms of carbon (graphite and diamond being the other two) that takes a spherical shape with a hollow interior. Buckyballs, named because they resemble the geodesic domes built by architect Buckminster Fuller, were discovered in 1985 among the byproducts of laser vaporization of graphite in which the carbon atoms are arranged in sheets. Though C60, referring to the number of carbon atoms that make up one sphere, is the most common fullerene, researchers have found stable, spherical carbon structures containing 70 atoms (C70), 120 (C120), 180 (C180), and others.
Robert F. Curl Jr. and Richard E. Smalley, both of Rice University in Houston, Texas and Harold W. Kroto of the University of Sussex in England, won the 1996 Nobel Prize for Chemistry for their discovery of buckminsterfullerene, the scientific name for buckyballs.
|
| |
|
|
When molecules or ions in the gas phase react and bond with H+ ions, the negative value of the free energy change of the reaction (-ΔG) is expressed as a thermochemical quantity. The protonation re-actions of molecules and ions have different values than proton affinity due to inclusion of chemical structure effects (entropy effect). This has an important role in the generation of multiply charged pro-tonated ions [M+nH]n+ in ESI.
|
| |
|
|
A detoxifying selenoenzyme responsible for preventing damage caused by oxidative stress. Its active site is the Se atom on a Se-Cysteine residue, and it functions by reducing hydroperoxides and organic peroxides.
|
| |
|
|
A Grignard Reagent is an alkyl- or aryl- magnesium halide. This reagent is important in the synthesis of carbon-carbon bonds in the Grignard reaction. Victor Grignard, of the University of Lyons, won the 1912 Nobel Prize in Chemistry for his discovery of Grignard reagents. Grignard reagents are formed by reacting alkyl or aryl halides with organomagnesium metal, conferring a negative charge on the terminal carbon, a rare occurrence. Bromides are most often used, as they work the fastest and are readily available among halides, iodide and chloride are also used, while fluoride is generally unreactive towards organomagnesium compounds. The Grignard reaction is exothermic and because of a oxide layer present on the magnesium, the start of the reaction is sometimes delayed. A crystal of iodine is often introduced to initiate the reaction.
|
| |
|
|
Hexabromocyclododecane (HBCD or HBCDD) is a brominated flame retardant.
Its primary application is in extruded (XPS) and expanded (EPS)
polystyrene foam that is used as thermal insulation in the building
industry. HBCD is highly efficient in this application so that very low
levels are required to reach the desired flame retardancy. Typical HBCD
levels in EPS are 0.7% and in XPS 2.5%.
|
| |
|
|
Hemin is an iron containing metalloporphyrin. Specifically, the Fe3+ oxidation product of heme is termed hemin. Hemin acts as a feed-back inhibitor on ALA synthase. Hemin also inhibits transport of ALA synthase from the cytosol (its' site of synthesis) into the mitochondria (its' site of action) as well as represses synthesis of the enzyme. Hemin is also known as a drug that is derived from processed red blood cells. Hemin for injection was known previously as hematin.
|
| |
|
|
one of two classes of natural acidic organic polymer that can be extracted from humus found in soil, sediment, or aquatic environments. The process by which humic acid forms in humus is not well understood, but the consensus is that it accumulates gradually as a residue from the metabolism of microorganisms. Humic acid is the humic fraction soluble in alkali, but not in acid. The other organic polymer is fulvic acid.
|
| |
|
Insoluble in water; the extent of insolubility; not readily absorbing water; resisting or repelling water, wetting, or hydration; or being adversely affected by water.
|
| |
|
|
Collision induced dissociation during ion formation in an APCI or ESI source. See also nozzle–skimmer dissociation.
|
| |
|
|
"The International Agency for Research on Cancer (IARC) is part of the World Health Organization (WHO).
IARC's
mission is to coordinate and conduct research on the causes of human
cancer, the mechanisms of carcinogenesis, and to develop scientific
strategies for cancer control. The Agency is involved in both
epidemiological and laboratory research and disseminates scientific
information through publications, meetings, courses, and fellowships."
It has collaborated to and published many highly recognized scientific publications.
Most publications are availaible from the webpage IARC Monographs Programme on the Evaluation of Carcinogenic Risks to humans  ,
"The IARC Monographs series publishes authoritative independent
assessments by international experts of the carcinogenic risks posed to
humans by a variety of agents, mixtures and exposures." ,
"The IARC Monographs series publishes authoritative independent
assessments by international experts of the carcinogenic risks posed to
humans by a variety of agents, mixtures and exposures."
IARC
distinguishes between four groups of compounds or physical factors
based on the existing scientific evidence for carcinogenicity: Standard IARC classification
(Source: IARC website )
|
| |
|
|
Iodothyronine deiodinase ( 1.97.1.10 ) (DI) is the
vertebrate enzyme responsible for the deiodination of the prohormone
thyroxine (T4 or 3,5,3',5'-tetraiodothyronine) into the biologically
active hormone T3 (3,5,3'-triiodothyronine) and of T3 into the inactive
metabolite T2 (3,3'-diiodothyronine). All known DI are proteins of
about 250 residues that contain a selenocysteine at their active site.
Three types of DI selenoenzymes are known, type II is essential for
providing the brain with the appropriate levels of T3 during the
critical period of development, and type III is essential for the
regulation of thyroid hormone inactivation during embryological
development.
|
| |
|
|
A primary high-energy ion beam for SIMS which is designed to act on a small area of the target sample. Typical beam dimensions are in the tens of nanometres.
|
| |
|
The part of the mass spectrometer used for sample ionization, since only particles which carry an electric charge can be analysed in a mass spectrometer. A variety of ion sources are in common use, each designed to ionize a specific class of atom or molecule.
See also: APCI, API, electron ionization (EI), chemical ionization, electrospray ionization, thermal ionization, plasma ionization etc.
|
| |
|
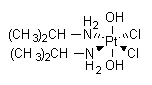 Iproplatin is a second generation metallodrug developed for cancer therapy. Iproplatin binds to and forms DNA crosslinks and platinum-DNA adducts,
resulting in DNA replication failure and cell death. Although less
prone to glutathione inactivation compared to cisplatin, resistance to
this agent has been observed in vitro due to repair of platination
damage by tumor cells.
IUPAC name: cis-dichlorobis(isopropylamine)-trans-dihydroxoplatinum(IV)
|
| |
|
In order to establish the relevance of a protein to an organism’s phenotype, it is important to be able to determine quantitative changes in its expression. This can be achieved by labelling with a compound (tag) which contains stable isotopes such as 2H. One batch of the organism is tagged with the heavy form of the label, containing the stable isotopes (2H), and another batch is tagged with the light form, containing 1H. The reactions are specific for cysteine residues within the proteins. The labelled samples are mixed and digested with trypsin and the isolated peptides are analysed by LC/MS/MS, enabling the relative abundances of each peptide pair, containing the light and heavy label, to be determined. ICAT is a commercial term.
|
| |
|
|
Thermal noise in electrical components originating from the thermal excitation of electrons. It occurs particularly in feedback resistors of electron multipliers.
|
| |
|
|
This is a form of direct introduction of elements from solid samples in the ICP-MS. LA can be used for analysis of single spots from 2D-gel electrophoresis, tissue samples from food, animals, and mineral or archeological samples, etc.
|
| |
|
|
A microanalytical technique designed to detect and characterise the elemental composition of small sections of surfaces. A visible range laser is focussed, with a microscope, on the area of the section to be analysed. This is followed by a pulsed high-energy laser beam to desorb and ionise the elements present in that area. Areas as small as 0.5 micron can be defined. The ions so formed are analysed with a TOF mass spectrometer.
|
| |
|
|
Classified as a blister agent (vesicant), Lewisite is named after the American military scientist, W. Lee Lewis, who produced this organoarsenic compound as a prototype chemical warfare (CW) agent in 1918. Because it was developed so late in World War I, Lewisite was never used in that conflict. The only military use of Lewisite known probably occurred in China during the Sino-Japanese conflict (ca. 1937-1942). Unlike mustard, which has delayed onset of clinical symptoms, the extreme irritation to eyes and skin begin almost immediately, with redness and blisters forming hours later. Significant exposure to Lewisite can cause blindness. Because of the rapid onset of pain, however, most exposures to Lewisite will result in less damage to the eyes as victims will attempt to avoid further contact by closing eyelids and avoiding the area. Lewisite, in its pure form, is an oily, colorless liquid, with no detectable odor. However, some have described impure batches of Lewisite as being of amber or dark brown color and having an odor of geraniums.
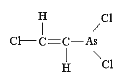 Depending on environmental conditions, Lewisite is a semi-persistent agent, and can penetrate a variety of rubber products, including those used in protective garments. Liquid at low temperatures (well below freezing), Lewisite was sometimes employed to mix with mustard to keep both CW agents solvent for use in winter conditions. Concentrations of Lewisite that can cause injury and death closely resemble those of mustard agent (also a vesicant). The median lethal concentration (LCt50) for Lewisite is about 1.5 grams-min/m3 for inhalation, and the median lethal dose (LD50) on the skin is estimated at 30mg/kg, or about 2.5 grams for an adult male of 180 pounds. The chemical formulation of Lewisite is relatively simple, and many countries, including those in the developing world, are capable of producing it in militarily significant quantities. |
| |
|
|
A statistical technique for estimating the best linear relationship
between two variables. The estimated line has the property that the sum
of the squares of the deviations from the line is a minimum, hence the
name least-squares analysis. This statistical technique is commonly
applied to the data from a comparison of methods experiment, taking the
test method values as the y-variable and the comparison method values as
the xvariable. The statistics calculated usually include the slope (b),
y-intercept (a), and standard deviation about the regression line, also
termed the standard error of the regression line (sy/x) and also called
the standard deviation of residuals (sres). These statistics provide
information about the proportional, constant, and random errors between
the methods, respectively.
|
| |
|
LC-MS is a hyphenated technique in which a liquid chromatograph is
coupled with a mass spectrometric analyzer. For coupling the LC
operated at atmospheric pressure with the high vacuum mass analyzer a
lot of different interfaces have been designed ranging from moving belt
over continuous-flow FAB, frit-FAB to ESI and APCI, with ESI and APCI
being the most successful interfaces.
|
| |
|
|
A method of extracting a desired component from a liquid mixture by bringing the solution into contact with a second liquid, the solvent, in which the component is also soluble, and which is immiscible with the first liquid, or nearly so. Separation is based on
different solubilities of the solute in the two phases. The
liquid-liquid extraction is a relatively gentle separation process and
is therefore suitable for unstable molecules
|
| |
|
|
The carbonate salt of lithium, a soft alkali metal, with antimanic and
hematopoietic activities. Lithium interferes with transmembrane sodium
exchange in nerve cells by affecting sodium, potassium-stimulated
adenosine triphosphatase (Na+, K+-ATPase); alters the release of
neurotransmitters; affects cyclic adenosine monophosphate (cAMP)
concentrations; and blocks inositol metabolism resulting in depletion
of cellular inositol and inhibition of phospholipase C-mediated signal
transduction. The exact mechanism through which lithium exerts its
mood-stabilizing effect has not been established. In addition, lithium
stimulates granulocytopoiesis and appears to increase the level of
pluripotent hematopoietic stem cells by stimulating the release of
hematopoietic cytokines and/or directly acting on hematopoietic stem
cells.
|
| |
|
 Lobaplatin (D-19466; 1,2-diammino-methyl-cyclobutaneplatinum(II)-lactate) is a new anticancer agent and a representative of the third-generation platinum compounds. The metallodrug lobaplatin consists of a nearly 50%/50% mixture of two diastereoisomers: (a) the SSS configuration (LP-D1); and (b) the RRS configuration (LP-D2) (see left.) . The compound has shown antitumor activity in human lung, gastric, testicular, and ovarian cancer xenografts, with incomplete cross-resistance to cisplatin in vitro and in vivo. Lobaplatin (D-19466; 1,2-diammino-methyl-cyclobutaneplatinum(II)-lactate) is a new anticancer agent and a representative of the third-generation platinum compounds. The metallodrug lobaplatin consists of a nearly 50%/50% mixture of two diastereoisomers: (a) the SSS configuration (LP-D1); and (b) the RRS configuration (LP-D2) (see left.) . The compound has shown antitumor activity in human lung, gastric, testicular, and ovarian cancer xenografts, with incomplete cross-resistance to cisplatin in vitro and in vivo.
|
| |
|
|
A solution of magnesium hydroxide with antacid and laxative properties.
Milk of magnesium exerts its antacid activity in low doses such that
all hydroxide ions that enter the stomach are used to neutralize
stomach acid. This agent exerts its laxative effect in higher doses so
that hydroxide ions are able to move from the stomach to the intestines
where they attract and retain water, thereby increasing intestinal
movement (peristalsis) and inducing the urge to defecate.
|
| |
|
|
The oxide salt of magnesium with antacid, laxative and vascular smooth
muscle relaxant activities. Magnesium combines with water to form
magnesium hydroxide which reacts chemically to neutralize or buffer
existing quantities of stomach acid; stomach-content and
intra-esophageal pH rise, resulting in a decrease in pepsin activity.
This agent's laxative effect is the result, in part, of osmotically
mediated water retention, which subsequently stimulates peristalsis. In
addition, magnesium ions may behave as calcium antagonists in vascular
smooth muscle.
|
| |
|
|
The magnesium salt of valproic acid (2-propylpentanoic acid) with
antiepileptic and potential antineoplastic activities. Magnesium
valproate dissociates in the gastrointestinal tract and is absorbed
into the circulation as magnesium ions and valproic acid ions; valproic
acid may inhibit histone deacetylases, inducing tumor cell
differentiation, apoptosis, and growth arrest. In addition, valproic
acid exerts an antiepileptic effect, likely by inhibiting enzymes that
catabolize the inhibitory neurotransmitter gamma-aminobutyric acid
(GABA) catabolism and so increasing concentrations of GABA in the
central nervous system (CNS). The presence of the magnesium in this
agent may contribute to its anticonvulsant activity and sedative
properties.
|
| |
|
|
Designates the kinetic energy spectrum of ions with a specific mass number and corresponds to the spectra of all product ions generated from a specific precursor ion. Using a reverse double-focusing mass spectrometer with a magnetic field --> electric field sequence, the precursor ions pass through a fixed magnetic field and are then scanned in the electric field to produce the spectrum.
|
| |
|
The Mathieu stability diagram is a graphical representation for reduced variables that incorporate the values of dc and ac voltages applied either to the four rods of a quadrupole mass filter or to the electrodes of an ion trap. The stability diagram illustrates areas of ion stability and ion instability and designates scan lines for the changes in those voltages so that the device can serve as an ion mass-to-charge ratio analyzer.
  
|
| |
|
|
The Mattauch-Herzog geometry is an instrumental design used in double-focusing mass spectrometers
when the electric field and magnetic field are arranged at deflection
angles of π/(4√2) and π /2 radians respectively.
|
| |
|
|
Designates rearrangement of hydrogen atoms, through a 6-membered ring, to a specific heteroatom. The term is also often used to describe the fragmentation that occurs simultaneously at the γ bond position of the heteroatom.
|
| |
|
Average time a substance remains in an animal body or an organ after rapid intravenous injection.
Note 1: Like clearance, its value is independent of dose in most cases.
Note 2: After an intravenous bolus
tr= Am/A
where tr is the MRT, A is the area under the plasma concentration-time curve, and Am is
the area under the moment curve.
Note 3: For a drug with one-compartment distribution characteristics, MRT equals the recipro-
cal of the elimination rate constant.
|
| |
|
Mercury, also called quicksilver, is a chemical element in the periodic table that has the symbol Hg (Latinized Greek: hydrargyrum, meaning watery or liquid silver) and atomic number 80. A heavy, silvery transition metal, mercury is one of five elements that are liquid at or near room temperature and pressure. (The others are the metals caesium, francium, and gallium, and the non-metal bromine.)
Mercury is used in thermometers, barometers and other scientific apparatus, though concerns about the element's toxicity have led to mercury thermometers being largely phased out in clinical environments in favour of alcohol-filled, digital or thermistor-based instruments. It remains in use in a number of other ways in scientific and scientific research applications, and in dental amalgam. Mercury is mostly obtained by reduction from the mineral, cinnabar.
Mercury occurs in deposits throughout the world and it is relatively harmless in an insoluble form, such as mercuric sulfide, but it is poisonous in soluble forms such as mercuric chloride or methylmercury.
|
| |
|
Enclosed and essentially self-sufficient (but not necessarily isolated) experimental environment or
ecosystem that is on a larger scale than a laboratory microcosm.
Note: A mesocosm is normally used outdoors or, in some manner, incorporated intimately
with the ecosystem that it is designed to reflect.
|
| |
|
|
Metalloproteins are proteins containing a metal ion cofactor that is required for the protein's biological activity. It is estimated that approximately half of all proteins contain a metal. Metalloproteins have many different functions in cells, such as enzymes, transport and storage proteins, and signal transduction proteins. In metalloproteins, metal ions are usually coordinated by nitrogen, oxygen or sulfur centres belonging to amino acid residues of the protein. In addition to donor groups that are provided by amino acid residues, a large number of organic cofactors function as ligands.
|
| |
|
|
Separation mode in capillary electrophoresis, separating according to the ability of apolar analytes to enter the (apolar) core of surface charged micelles
|
| |
|
|
- the movement of ionic solutes between opposite electrodes during electrophoresis.
|
| |
|
|
- the speed with which ionic solutes move through a capillary during electrophoresis.
|
| |
|
|
The maximum concentration of residue resulting from the use of a veterinary drug (expressed in mg/kg or mg/kg on a fresh weight basis) that is acceptable in or on a food. It is based on the type and amount of residue considered to be without toxicological hazard for human health as expressed by the Acceptable Daily Intake (ADI), or on the basis of a temporary ADI that utilizes an additional safety factor. It also takes into account other relevant public health risks as well as food technological aspects and estimated food intakes. When establishing an MRL, consideration is also given to residues that occur in food of plant origin and/or the environment. Furthermore, the MRL may be reduced to be consistent with good practices in the use of veterinary drugs and to the extent that practical analytical methods are available.
|
| |
|
|
A nanoparticle is a microscopic particle whose size is measured in nanometres. Nanoparticles are thus larger than angstroms (Ĺ), but smaller than micrometres. At the small end of the size range, nanoparticles are often referred to as clusters (cluster (physics)).
Metal, dielectric, and semiconductor nanoparticles have been formed, as well as hybrid structures (e.g., core-shell nanoparticles). Nanospheres, nanorods, and nanocups are just a few of the shapes that have been grown. Semiconductor quantum dots and nanocrystals are types of nanoparticles.
Nanoparticle characterization is necessary to establish understanding and control of nanoparticle synthesis and applications. Characterization is done by using a variety of different techniques, mainly drawn from materials science. Common techniques are electron microscopy, Atomic force microscopy, x-ray photoelectron spectroscopy, fourier transform infra red spectroscopy (FTIR).
Nanoparticle research is currently an area of intense scientific research, due to a wide variety of potential applications in biomedical, optical, and electronic fields.
In biomedical applications nanoparticles are used as drug carriers or imaging agents. For this purpose the nanoparticle may have a hollow structure providing a central reservoir that can be filled with anticancer drugs, detection agents, or chemicals, known as reporters, that can signal if a drug is having a therapeutic effect. The surface of a nanoparticle can also be adorned with various targeting agents, such as antibodies, drugs, imaging agents, and reporters. Most nanoparticles are constructed to be small enough to pass through blood capillaries and enter cells. |
| |
|
The term nanospray describes a design for a miniaturized electrospray ionization source using a pulled and coated glass capillary as the spray tip. This design achieves a flow rate of 20–50 nL/min, much lower than the usual electrospray ionization source.
  
|
| |
|
The term nanotechnology applies to materials, structures and technologies with one thing in common: the creation or presence of at least one spatial dimension smaller than a few hundred nanometers. This includes the production of nanoparticles and the creation of nanostructures, which in turn make it possible to produce products with new or improved properties. Examples include starting materials for textiles that absorb UV radiation, water-repellant surface coatings for the textile and automotive industries and coatings that are more scratch resistant. |
| |
|
|
Native mass spectrometry (native MS) is defined as the process whereby
large biomolecules and complexes thereof can be transferred from a
three-dimensional, functional existence in a condensed liquid phase to
the gas phase via the process of electrospray ionization mass
spectrometry (ESI-MS). The experimental conditions where this is
possible are so mild that non-covalent interactions, the native state
and the associated biological action and functionality of the
molecule/complex are largely preserved.
|
| |
|
|
- A malleable, silvery metal with excellent resistance to corrosion. Used in the production of alloys, electroplating, catalysts, welding rods and coinage and can be found in electronic equipment, construction materials, aerospace equipment and consumer goods such as alkaline batteries, paints and ceramics. Hazard: Ingestion of nickel may cause nausea, vomiting and diarrhea. Hypersensitivity to nickel is common and can cause allergic contact dermatitis, pulmonary asthma, and conjunctivitis.
|
| |
|
|
The Nier-Johnson geometry is an instrumental design used in double-focusing mass spectrometers when the electric field and magnetic field are arranged at deflection angles of π/2 and π/3 radians respectively.
|
| |
|
|
An experimental procedure that is designed to measure a specific parameter, whereby the parameter is defined by this measurement procedure.
For example: Total alkalinity is operationally defined as the alkalinity neutralized by titration with a strong acid to the carbonic acid equivalence point. (IT = incremental titration, DIS = dissolved, TOT = total)
|
| |
|
|
An oral preparation of picoplatin, a third generation platinum compound
with antineoplastic activity. Designed to overcome platinum drug
resistance, picoplatin alkylates DNA, forming both inter- and
intra-strand cross-linkages, resulting in inhibition of DNA replication
and RNA transcription and the induction of apoptosis.
Because of the increase in steric bulk around the platinum center,
there is a relative reduction in the inactivation of picoplatin by
thiol-containing
species such as glutathione and metallothionein in comparison to
cisplatin.
|
| |
|
 Oxaliplatin (trans-1-diaminocyclohexane oxalatoplatinum) is a platinum-based chemotherapy metallodrug in the same family as cisplatin and carboplatin. Compared to cisplatin the two amine groups are replaced . with 1,2-diaminocyclohexane (DACH) and with an oxalate ligand as a
'leaving group' for improved antitumour activity. A 'leaving group' is an atom or a group of atoms that
is displaced as a stable species taking with it the bonding electrons.
After displacement of the labile oxalate ligand leaving group, active
oxaliplatin derivatives, such as monoaquo and diaquo DACH platinum,
alkylate macromolecules, forming both inter- and intra-strand
platinum-DNA crosslinks, which result in inhibition of DNA replication
and transcription and cell-cycle nonspecific cytotoxicity. The DACH
side chain appears to inhibit alkylating-agent resistance.
CAS number: 61825-94-3
Chemical formula C8H14N2O4Pt
Molecular weight 397.3
|
| |
|
|
The oxidation number of an element in any chemical entity is the number of charges which would remain on a given atom if the pairs of electrons in each bond to that atom were assigned to the more electronegative member of the bond pair. The oxidation (Stock) number of an element is indicated by a roman numeral placed in parentheses immediately following the name (modified if necessary by an appropriate ending) of the element to which it refers. The oxidation number may be positive, negative or zero. Zero, not a roman numeral, is represented by the usual cipher, 0. The positive sign is never used. An oxidation number is always positive unless the minus sign is explicitly used. Note that it cannot be non-integral (see also mixed valency). Non-integral numbers may seem appropriate in some cases where a charge is spread over more than one atom, but such a use is not encouraged. In such ambiguous cases, the charge number, which designates ionic charge, can be used. A charge (Ewens-Bassett) number is a number in parentheses written without a space immediately after the name of an ion, and whose magnitude is the ionic charge. Thus the number may refer to cations or anions, but never to neutral species. The charge is written in arabic numerals and followed by the sign of the charge. In a coordination entity, the oxidation number of the central atom is defined as the charge it would bear if all the ligands were removed along with the electron pairs that were shared with the central atom. Neutral ligands are formally removed in their closed-shell configurations. Where it is not feasible or reasonable to define an oxidation state for each individual member of a group or cluster, it is again recommended that the overall oxidation level of the group be defined by a formal ionic charge, the net charge on the coordination entity.
|
| |
|
|
Polyacrylamide gel electrophoresis (PAGE) is a technique for the seperation of proteins in which a polyacrylamide gel is used as a supporting matrix through which proteins can migrate and electrophoresis is used as the principle for separation. Separation occurs, because small molecules can manuver through the polyacrylamide gel faster than big molecules.
|
| |
|
|
procedure whereby a spectrum, generated by peak synthesis, is adjusted to match a measured spectrum
|
| |
|
Phytochelatins (PCs) are oligomers of glutathione, produced by the enzyme phytochelatin synthase (PCS). They are found in plants, fungi, nematodes and all groups of algae including cyanobacteria and are important for heavy metal detoxification.
PCs have the general structure of (g-glutamyl-cysteinyl)n-glycine (n=2-11) and are therefore abbreviated as PC2 to PC11.
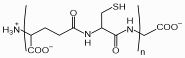
Chemical structure of phytochelatin with n=2-11
Phytochelatins act as chelators, binding metal ions through thiol coordination and by this way,
limit damage to metabolic processes in reducing the cytotoxic free metal
ion. Because phytochelatin synthase uses glutathione with a blocked thiol group in the synthesis of phytochelatin, the presence of heavy metal ions that bind to glutathione causes the enzyme to work faster. Therefore the amount of phytochelatin increases when the cell needs more phytochelatin to survive in an environment with high concentrations of metal ions. Its widespread presence in microorganisms suggests that PC-based metal detoxification might be an ancient type of defense mechanism established in micro-algae or micro-fungi.
|
| |
|
|
A new generation organic platinum analog with an extended spectrum of
antineoplastic activity. Designed to overcome platinum drug resistance,
picoplatin alkylates DNA, forming both inter- and intra-strand
cross-linkages, resulting in inhibition of DNA replication and
transcription, and the induction of apoptosis.
|
| |
|
|
(in biology)
1. Fluid component of blood in which the blood cells and platelets are suspended (blood plasma).
2. Fluid component of semen produced by the accessory glands, the seminal vesicles, the prostate, and in bulbo-urethral glands.
3. Cell substance outside the nucleus, i.e. the cytoplasm.
(in physics)
Gas, ionized to a certain degree
|
| |
|
Any material entering the environment that has undesired effects.
|
| |
|
|
With MALDI, this term designates the phenomenon occurring when the excessive internal energy of ions themselves or collisions with free gas cause dissociation immediately after the ions generated by laser illumination exit the high speed field region. Since the ions decay after leaving the ion source but before reaching the collector, this term corresponds to metastable dissociation.
|
| |
|
|
Potassium hexachloroplatinate is the inorganic compound with the formula K2PtCl6.
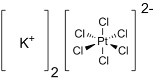
It is a yellow solid that is an example of a comparatively
insoluble potassium salt. The salt features the hexachloroplatinate(IV)
dianion, which has octahedral coordination geometry.It is a raw material for the synthesis of Pt-based drugs.
|
| |
|
|
Potassium tetrachloroplatinate(II) is the chemical compound with the formula K2PtCl4.
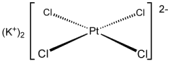
This reddish orange salt is an important reagent for the preparation of other coordination complexes of platinum. It consists of potassium cations and the square planar dianion PtCl42−.
It is a raw material for the synthesis of Pt-based drugs such as cis-platin.
|
| |
|
|
Standard that is designated or widely acknowledged as having the highest metrological qualities and whose value is accepted without reference to other standards of the same quantity, within a specified context.
|
| |
|
Propylation is a derivatization technique in which the analyte species
is transformed by adding propyl groups. For this alkylation either a
Grignard reagent based on propylmagnesium or sodium tetrapropylborate
can be used. Propylation is especially usefull for the analysis of
methyl- or ethyl-species, for which methylation or ethylation would
destroy molecular information.
|
| |
|
|
Arrangements of four electrostatic rods (Quad) in which ions with a desired quotient mass/ charge are made to describe a stable path under the effect of a static and a high- frequency electric quadrupole field applied to the rods, and are then detected. Ions with a different mass/ charge are separated from the detected ions because of their unstable paths deflecting them from the axis towards the detector.
|
| |
|
|
Chemical analysis designed to identify the components of a substance or mixture.
|
| |
|
|
Limiting droplet size at which self-fragmentation will occur with static charge droplets generated in ESI or other ionization processes. Self-fragmentation occurs when the Coulombic repulsion force generated by the excess charge in a droplet exceeds the surface tension maintaining the droplet. Vaporization of electrons and ions from the static charge droplet surface occurs even with droplets having large sizes. As the size becomes even smaller, the surface tension begins to squeeze the droplet and promote further vaporization.
|
| |
|
The relative abundance of an ion is the measured intensity for the ion beam at that designated m/z value. To be precise, ion beams have intensities, and ions have abundances. Relative abundance is a term related to the practice of assigning the most abundant ion in a measured and plotted mass spectrum a relative abundance of 100% and normalizing all other ion abundances to that value.
  
|
| |
|
|
In mass spectrometry relative intensity designates the ratio between the intensity of the ion beam for a certain mass and the maximum intensity of the ion beam. Normally this ratio is expressed using the heights of each peak on a spectrum with the highest peak defined as 100. Another method defines the total ion quantity or the ion quantity in a specific region as 100. The peak height method is also called the pattern coefficient while the ion quantity method is called the %? method. With the ion peak intensity terms for ion quantity (abundance), ion peak strength (intensity), peak height (height) and peak surface area (area) are also used.
|
| |
|
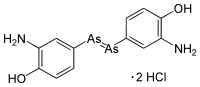 Arsphenamine, also known as Salvarsan and 606, is a drug containing arsenic that was used to treat syphilis and trypanosomiasis. The organoarsenic compound was the first modern chemotherapeutic agent. Arsphenamine was marketed under the trade name Salvarsan in 1910. It was also called 606, because it was the 606th compound synthesized for testing. Salvarsan was the first organic anti-syphillitic, and a great improvement over the inorganic mercury compounds that had been used previously. A more soluble (but slightly less effective) arsenical compound, Neosalvarsan, (neoarsphenamine), became available in 1912. These arsenical compounds came with considerable risk of side effects, and they were supplanted as treatments for syphilis in the 1940s by penicillin. Arsphenamine, also known as Salvarsan and 606, is a drug containing arsenic that was used to treat syphilis and trypanosomiasis. The organoarsenic compound was the first modern chemotherapeutic agent. Arsphenamine was marketed under the trade name Salvarsan in 1910. It was also called 606, because it was the 606th compound synthesized for testing. Salvarsan was the first organic anti-syphillitic, and a great improvement over the inorganic mercury compounds that had been used previously. A more soluble (but slightly less effective) arsenical compound, Neosalvarsan, (neoarsphenamine), became available in 1912. These arsenical compounds came with considerable risk of side effects, and they were supplanted as treatments for syphilis in the 1940s by penicillin.
Source: Wikipedia
|
| |
|
|
Selenocysteine is an amino acid that is present in several selenoproteins (seleno-enzymes) in plants and animals (for example glutathione peroxidases, tetraiodothyronine 5' deiodinases, thioredoxin reductases, formate dehydrogenases, glycine reductases and some hydrogenases).
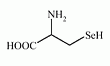 Selenocysteine has a structure similar to cysteine, but with an atom of selenium taking the place of the usual sulfur. Selenium is incorporated into amino acid sequences of selenoproteins by the specific codon to SeCys residue. |
| |
|
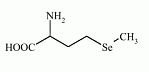 Selenomethionine is an selenoamino acid (amino acid containing selenium). The L-isomer of selenomethionine, known as Se-met, is a common natural food source of selenium. It can not be synthesized by higher animals, but can be obtained from plant material.
Chemical formula: C5H11NO2Se
IUPAC name: 2-amino-4-methylselanyl-butanoic acid
|
| |
|
|
A selenoprotein is any protein that includes a selenocysteine residue. Selenoproteins exist in all major forms of life, eukaryote, eubacteria and archaea. Among eukaryotes, selenoproteins appear to be common in animals, but rare or absent in other phyla (one has been identified in the green alga Chlamydomonas, but none in other plants or in fungi). Among eubacteria and archaea, selenoproteins are only present in some lineages, while they are completely absent in many other phylogenetic groups.
|
| |
|
|
A glycoprotein synthesized mostly by the liver that serves as the main plasma selenoprotein. Its role is thought to involve extracellular antioxidant activity and Se transport.
|
| |
|
Poisoning due to excessive intake of Se. Symptoms include hair and nail loss, skin lesions, damage to the nervous, immune and reproductive systems, convulsions, paralysis, gastrointestinal and circulatory
disturbances.
|
| |
|
|
A sheath flow interface is a method for coupling capillary
electrophoresis to electrospray ionization that uses a coaxial flow of
makeup liquid that is introduced through a tube that is concentric with
the separation capillary.
|
| |
|
|
Analytical separation technique, often used to characterize proteins or mixtures, that uses a charged gel environment through which molecules of varying sizes and electric charges migrate from one pole to the other. Unlike gel-filtration chromatography, larger molecules move more slowly than smaller molecules because their migration rate is not dependent on diffusion into and out of particles. The SDS detergent denatures and binds to proteins, aiding in their separation.
|
| |
|
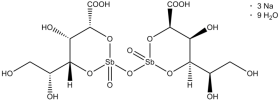 Sodium stibogluconate belongs to the class of medicines known as the pentavalent antimonials used to treat leishmaniasis. Sodium stibogluconate is sold in the UK as Pentostam (manufactured by GlaxoSmithKline) and is only available for administration by injection. Widespread resistance has limited the utility of sodium stibogluconate, and in many parts of the world, amphotericin or miltefosine are used instead. Sodium stibogluconate belongs to the class of medicines known as the pentavalent antimonials used to treat leishmaniasis. Sodium stibogluconate is sold in the UK as Pentostam (manufactured by GlaxoSmithKline) and is only available for administration by injection. Widespread resistance has limited the utility of sodium stibogluconate, and in many parts of the world, amphotericin or miltefosine are used instead.
|
| |
|
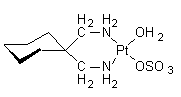 Spiroplatin is a second generation metallodrug and analgue for cisplatin, developed for cancer therapy. Spiroplatin induces DNA cross-links, thereby
inhibiting DNA replication and RNA and protein synthesis. Similar to
other platinum compounds, this agent has been shown to be mutagenic and
carcinogenic.
IUPAC name: aqua-1,1-bis(aminomethyl)-cyclohexanesulfatoplatinum(II)
|
| |
|
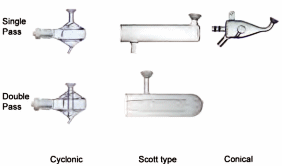 The spray chamber is part of the sample introduction system for liquid samples of a spectroscopic source such as a flame or plasma. The function of the spray chamber is to filter the aerosol produced by the nebulizer (primary/secondary aerosol) so that only the smallest reach the source (tertiary aerosol). The most commonly used spray chambers in plasma spectrochemistry are the cyclonic, barrel (or Scott-type), and conical (see figure). The spray chamber is part of the sample introduction system for liquid samples of a spectroscopic source such as a flame or plasma. The function of the spray chamber is to filter the aerosol produced by the nebulizer (primary/secondary aerosol) so that only the smallest reach the source (tertiary aerosol). The most commonly used spray chambers in plasma spectrochemistry are the cyclonic, barrel (or Scott-type), and conical (see figure).
The cyclonic and Scott-type spray chambers are available as both single and double pass (or baffled) versions. The double-pass mode acts as a secondary filter to further reduce the mean droplet site distribution (reducing the aerosol transport efficiency and reducing the noise level). The conical spray chamber uses an impact bead to break-up larger droplets. In all three designs gravity is used to remove the larger drops from the transport gas stream and divert them to the drain. Total transport efficiency is typically below 5 %. In the cyclonic spray chamber this action is assisted by centrifugal forces.
|
| |
|
|
Often called Student’s t-test. A statistical
test of significance in which the difference between two mean values is
tested. The null hypothesis is that there is no difference between the
two means. The test is carried out by calculating a t-value, then
comparing the calculated t-value with a critical t-value which is
obtained from a statistics table. If the calculated t-value is greater
than the critical t-value, the null hypothesis is rejected; this means
that a statistically significant or real difference exists between the
mean values being compared. If the calculated t-value is less than the
critical t-value, the null hypothesis stands, therefore no difference
has been observed between the two mean values.
|
| |
|
|
Used in proficiency testing to
designate the correct value, usually estimated by the mean of all
participant responses, after removal of outliers, or by the mean
established by definitive or reference methods.
|
| |
|
|
Tetramethylammonium hydroxide (TMH) is an effective etchant and chelating agent ideal for removing residue and contaminants from semiconductor and electronic components. It also is often used to solubilze biological materials by alkaline hydrolysis.
Molecular formula: C4H13NO.5H2O
MW: 181.22972
|
| |
|
|
A protein containing a Se-Cysteine residue that regulates intracellular redox reactions (including reduction of thioredoxin) and factors into repair mechanisms in DNA synthesis.
|
| |
|
Tin is a chemical element in the periodic table that has the symbol Sn (Latin: stannum) and atomic number 50. This silvery, malleable poor metal that is not easily oxidized in air and resists corrosion is found in many alloys and is used to coat other metals to prevent corrosion. Tin is obtained chiefly from the mineral cassiterite, where it occurs as an oxide. It is the classic alloying metal to make bronze.
Elemental tin is an essential nutrient, needed in very small amounts. However, certain organic tin compounds, organotin, such as triorganotins (see tributyltin oxide) are toxic and are used as industrial fungicides and bactericides.
|
| |
|
|
A TDI is an estimate of the amount of a substance in air, food or drinking
water that can be taken in daily over a lifetime without appreciable health
risk. TDIs are calculated on the basis of laboratory
toxicity data to which
uncertainty
factors are applied.
TDIs are used for substances that do not have a reason to be found in food (as
opposed to substances that do, such as additives, pesticide residues or
veterinary drugs in foods- see also Acceptable Daily Intake : ADI).
|
| |
|
|
A transition element is an element whose atom has an incomplete d-sub-shell, or which gives rise to a cation or cations with an incomplete d-sub-shell.
The First Transition Series of elements is Sc, Ti, V, Cr, Mn, Fe, Co,
Ni, Cu. The Second and Third Transition Series are similarly derived:
these include the lanthanoids (lanthanides) and actinoids (actinides)
respectively which are designated inner (or f) transition elements of their respective Periods in the Periodic Table.
|
| |
|
|
A mixture formed by the hydrolysis of a peptide or protein, or a mixture of these, using the enzyme trypsin. Cleavage of the peptide chain occurs on the C-terminal side of arginine and lysine residues to give a series of peptides which can be identified by sequencing or mass mapping to lead to identification of the original protein. The technique is used extensively in proteomics.
|
| |
|
|
"As the Center for Disease Control and Prevention (CDC) is
recognized as the lead federal agency for protecting the health and
safety of people - at home and abroad, providing credible information
to enhance health decisions, and promoting health through strong
partnerships. CDC serves as the national focus for developing and
applying disease prevention and control, environmental health, and
health promotion and education activities designed to improve the
health of the people of the United States."
"CDC, located in Atlanta, Georgia, USA, is an agency of the Department of Health and Human Services."
(Source: CDC website )
|
| |
|
|
A vitamin synthesized by microorganisms and conserved in
animals in the liver. Deficiency or collective uptake of vitamin B-12
leads to pernicious anemia. It consists of cobalamin, a substituted
corrin-Co(III)
complex in which the cobalt atom is bound to the four nitrogen atoms of
the corrin ring, an axial group A and 5,6-dimethylbenzimidazole.
Various forms of the vitamin are known with different A groups such as
A = CN,
cyanocobalamin; R = OH, hydroxocobalamin; R = CH3, methylcobalamin; R =
adenosyl, coenzyme B-12.
|
| |
|
An immunochemical method for identifying proteins in a complex mixture, proteins separated by electrophoresis are transferred (blotted) from the gel medium to a protein-binding nitrocellulose or polymeric membrane; the transferred proteins are then detected by their relative binding to labeled antibodies.
|
| |
|
|
WHAM (Windermere Humic-Aqueous Model) is designed to
calculate equilibrium chemical speciation in surface and ground waters, sediments and
soils. The model is especially suitable for problems where the chemical speciation is
dominated by organic matter (humic substances). WHAM combines Humic Ion-Binding Model V
with a simple inorganic speciation code for aqueous solutions. Precipitation of aluminium
and iron oxides, cation-exchange on an idealized clay mineral, and adsorption-desorption
reactions of fulvic acid are also taken into account. The importance of ion accumulation
in the diffuse layers surrounding the humic molecules is emphasized. Model calculations
are performed with a BASIC computer code running on a Personal Computer.
|
| |
|