|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
|
Process in which a substance is converted to simpler products by physical or chemical mechanisms: examples include hydrolysis and photolysis
|
| |
|
|
Process in which a substance in the environment is modified by nonbiological mechanisms.
|
| |
|
|
A method of chemical analysis that bases characterization completely on standards defined in terms of physical properties.
|
| |
|
|
Substance that enters and is retained inside a solid or semisolid matrix (absorbent).
|
| |
|
|
A solid that assimilates a gas, liquid, or solute into its interior (compare with adsorbent and sorbent).
|
| |
|
The process by which a substance or a xenobiotic is brought into a body (human or animal).
Also: incorporated into the structure of a mineral.
|
| |
|
|
This term is used to describe the relative occurrence of an ion. A mass spectrum is a plot of the ion abundances against the m/z values determined and is normalised to the most abundant ion. This term should be distinguished from intensity which, while appropriate for the signal of the individual ion peak detected, is not used in spectra.
|
| |
|
The measure of an analyzer’s ability to separate adjacent peaks differing greatly in
intensity.
|
| |
|
|
Plasma
created by a power supply or function generator of alternating
polarity. Typically, an arc excited at twice the frequency of the
electrical grid (50 Hz except North America, which is 60 Hz). While not
continuous, the period of low current as the polarity of the driving
voltage commutes (switches sign) is brief compared to the overall
experiment time.
|
| |
|
|
Analytical technique of performing static extractions at elevated temperatures and pressures for enhanced extraction efficiency and sample through-put.
|
| |
|
The accelerating voltage is applied to the source of a mass spectrometer to move ions formed in the source into the mass analyzer of the instrument. This accelerating voltage can be a few tens of volts in quadrupole mass spectrometers to several thousand volts in sector instruments or in time-of-flight mass spectrometers.
  
|
| |
|
The degree of conformity with a standard, or the degree of perfection attained in a measurement. Accuracy relates to the quality of a result, and is distinguished from precision, which relates to the quality of the operation by which the result is obtained.
|
| |
|
The accurate mass (or exact mass) of an ion of specified isotopic composition is calculated by summation of the exact masses of the constituent atoms. Conversely, the empirical formula of an ion can be deduced from the measured accurate mass of the ion if the ion mass is low enough to limit the number of formulaic possibilities and if the exact mass value is known accurately enough.
  
|
| |
|
|
A synthetic N-acetyl derivative of the endogenous amino acid
L-cysteine, a precursor of the antioxidant enzyme glutathione.
Acetylcysteine regenerates liver stores of glutathione. This agent also
reduces disulfide bonds in mucoproteins, resulting in liquification of
mucus. Some evidence suggests that acetylcysteine may exert an
anti-apoptotic effect due to its antioxidant activity, possibly
preventing cancer cell development or growth. In addition,
acetylcysteine has inhibited viral stimulation by reactive oxygen
intermediates, thereby producing antiviral activity in HIV patients
|
| |
|
|
In treating samples, the use of acid to chemically decompose a material into its simpler constituents (usually soluble) thereby releasing the analytes for subsequent analysis.
|
| |
|
|
Determined by SnCl2 reduction on acidified samples, includes inorganic complexes, labile organic associations, elemental mercury, and labile particulate mercury. Doesn't measure organic forms (C-Hg bound) of mercury such as methylmercury. Same as reactive mercury.
|
| |
|
|
Liquid drainage from bituminous coal mines containing a high concentration of acidic sulfates, especially ferrous sulfate.
|
| |
|
|
Mercury that passes through a 0.45 µm membrane filter after the sample is acidified to pH1-2.0 with nitric acid (EPA 1984). Strongly sorbed Hg is not measured, but all toxic forms as well as some non-toxic forms are measured.
|
| |
|
Sulfides in sediments that liberate hydrogen sulfide on reaction with cold dilute acid (mainly FeS or MnS in sediments). AVS is used as a parameter together with SEM (simultaneously extractable metals) to predict toxicity of sediments.
AVS may be chemically available by acid extraction without being bioavailable. Thus, AVS can reduce metal toxicity by binding metals in anoxic soils or sediments, thereby rendering them unavailable to most living organisms.
|
| |
|
|
An element which is any of the second (5f) series of f-block elements or inner transition elements commencing with actinium at atomic number 89 and ending with lawrencium at atomic number 103.
|
| |
|
1. Concentration of a substance in air, soil, water or other defined medium at which specified emergency counter-measures, such as the seizure and destruction of contaminated materials, evacuation of the local population or closing down the sources of [pollution], are to be taken.
2. Concentration of a [pollutant] in air, soil, water or other defined medium at which some kind of preventive action (not necessarily of an emergency nature) is to be taken. (IUPAC)
|
| |
|
|
Movement of a substance across a cell membrane against an electrochemical gradient, in the direction opposite to normal diffusion and requiring the expenditure of energy
|
| |
|
in chemistry: The thermodynamically effective concentration of a chemical species or component. In solutions with high ionic strengths, ions interact with each other and are not totally independent chemical units. The interactions affect the boiling point, freezing point, and other properties of the solution. The effective concentration (activity) of an ion in a high ionic strength solution is usually less than the total number of moles in the solution. The activity of an ion is calculated by multiplying its molal concentration by its activity coefficient. In very dilute solutions, activities and molal concentrations are essentially equal.
|
| |
|
Contact with a substance that occurs once or for only a short time (up to 14
days [for humans]).
Source: ATSDR Glossary of Terms
|
| |
|
Any poisonous effect produced within a short period of time following
exposure, usually up to 24-96 hours, resulting in biological harm and
often death.
|
| |
|
|
Adamsite (DM) is an organoarsenic compound developed near the conclusion of World War I. The chemical warfare compound is solid when pure, and has been used as an aerosol dispersed by thermal grenades or smoke generators. Its effect are: severe irritation of the eyes, nose, and throat. If the agent is inhaled for 1-2 minutes, tightness of the chest and headache are experienced. The headache develops into general nausea, which can result in vomiting in approximately three minutes. Under concentrations expected to occur under combat conditions, fatalities are not expected; however, these compounds can be fatal at higher concentrations.
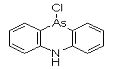 DM was discovered by German scientists in 1913 (Ger. pat. appl. 281049, July 1913 to F. Bayer and Co.), but was never used by Germany. It was independently discovered by Major Robert Adams working at the university of Illinois and also by a British team, both at the beginning of 1918. DM was produced, but not used, by the Americans at the end of the war; Franke states that "according to very incomplete reports [it] was used by the Italian Army." It was produced by many nations for use as a riot control agent until it was superseded by alpha-chloroacetophenone (CN) and similar tear agents. It was also found to be effective as a pesticide against marine borers, which kept in production for years. By 1920, gas mask filters had been improved to protect against aerosol particles, which may account for the termination of this line of development.
Names: DM, 10-Chloro-5,10-dihydrophenarsazine, Adamsite
Molecular formula: C12H9AsClN
CAS Registry Number: 578-94-9
|
| |
|
A new chemical species AB, each molecular entity of which is formed by direct combination of two separate molecular entities A and B in such a way that there is change in connectivity, but no loss, of atoms within the moieties A and B. Stoichiometries other than 1:1 are also possible, e.g. a bis-adduct (2:1). An intramolecular adduct can be formed when A and B are groups contained within the same molecular entity. This is a general term which, whenever appropriate, should be used in preference to the less explicit term complex. It is also used specifically for products of an addition reaction.
source: IUPAC Gold Book
|
| |
|
An electrically charged species formed by non-covalent attachment between an ion and a neutral species. These are most commonly observed in desorption and API and CI ion sources where a molecule of a component in the solvent or reagen gas remains attached to the ion.
Source: A. Mallet, S. Down, Dictionary of Mass Spectrometry, Wiley, 2010
|
| |
|
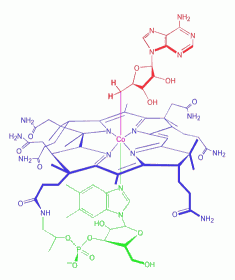 Adenosylcobalamin
is a cobalamin derivative in which the ß-substituent is deoxyadenosyl
(see the red ligand in left figure). It is one of two metabolically
active forms synthesized upon ingestion of vitamin B12 and is the
predominant form in the liver; it acts as a coenzyme in the reaction
catalyzed by methylmalonyl-CoA mutase. Adenosylcobalamin
is a cobalamin derivative in which the ß-substituent is deoxyadenosyl
(see the red ligand in left figure). It is one of two metabolically
active forms synthesized upon ingestion of vitamin B12 and is the
predominant form in the liver; it acts as a coenzyme in the reaction
catalyzed by methylmalonyl-CoA mutase.
Cobalamins are coenzymatically active forms of vitamin B12
(cyanocobalamin) which is a water-soluble vitamin and a
nutrient essential for all cells. Cobalamin is a
substituted corrin-Co(III) complex (see blue structure in left figure)
in which the cobalt atom is bound to the four nitrogen atoms of the
corrin ring, an axial group R and 5,6-dimethylbenzimidazole (DMBI, see
green part in left figure). The latter is linked to the cobalt by the
N-3 nitrogen atom and is bound to the C-1 carbon of a ribose molecule
by the N-1 nitrogen atom. Various forms of the vitamin are known with
different R groups such as R = CN, cyanocobalamin; R = OH,
hydroxocobalamin; R = CH3, methylcobalamin; R = adenosyl, coenzyme B-12
(shown here).
|
| |
|
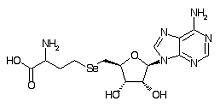 Adenosylselenohomocysteine is a metabolite within the selenium metabolic pathway.
Formula C14H20N6O5Se
Mass 432.066
|
| |
|
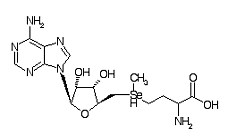 Adenosylselenomethione is a metabolite within the Se metabolic pathway.
Formula C15H24N6O5Se
CAS Registry No. 5134-38-3
Chemical Synonym: AdoSeMet
Molecular Weight: 447.349 g/mol
|
| |
|
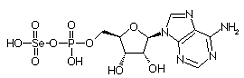 Adenylylselenate is the first metabolite in the metabolism of Se in plants. The first step is the activation of selenate by ATP sulfurylase to form 5'-adenylylselenate Adenylylselenate is the first metabolite in the metabolism of Se in plants. The first step is the activation of selenate by ATP sulfurylase to form 5'-adenylylselenate
|
| |
|
|
An estimate of the amount of a substance in food or drinking water, expressed on a body-weight basis, that can be ingested daily over a lifetime without appreciable risk (standard human = 60 kg). The ADI is listed in units of mg per kg of body weight.
|
| |
|
Process whereby an electron is removed from an atom, ion, or molecule in its lowest energy state
to produce an ion in its lowest energy state.
|
| |
|
|
A solid material that removes chemical species from liquids or gases by causing them to adhere onto its surface (compare with absorbent and sorbent).
|
| |
|
|
Adsorption is the enrichment (positive adsorption. or briefly, adsorption) or depletion (negative adsorption) of one or more components in an interfacial layer.
|
| |
|
|
A chromatographic method in which the molecules are retained through the interaction of the polar functional groups of the sample components and the functional groups on the surface of the packing material.
|
| |
|
Adsorption complex is a molecular term used to denote the entity constituted
by the adsorbate and that part of the adsorbent to which it is bound.
|
| |
|
A change in body function or cell structure that might lead to disease or
health problems.
Source: ATSDR Glossary of Terms
|
| |
|
|
Particles, solid or liquid, suspended in a gas typically air.
|
| |
|
|
A technique to dilute a sample after nebulization for sample introduction into an ICP. A flow of argon gas is added to the sample aerosol generated by the nebulizer before the aerosol is reaching the torch injector. When keeping the total flow to the injector constant, this has the effect of reducing the sample’s solvent loading to the plasma, so it can tolerate much higher total dissolved solids.
|
| |
|
|
Aerosol matrix-assisted laser desorption ionization is a variant of
MALDI in which a mixture of matrix and analyte is nebulized and the
resulting particles irradiated with a laser to form ion.
|
| |
|
|
An abbreviation for asymmetrical flow field-flow fractionation
|
| |
|
|
- a technique in which a biospecific adsorbent is prepared by coupling a specific ligand (such as an enzyme, antigen, or hormone) for the macromolecule of interest to a solid support (or carrier). This immobilised ligand will interact only with molecules that can selectively bind to it. Molecules that will not bind elute unretained. The retained compound can later be released in a purified state.
|
| |
|
The Agency for Toxic Substances and Disease Registry (ATSDR)
is an agency of the U.S. Department of Health and Human Services (HHS). As
mandated by the federal superfund law, the agency assesses health risks from
hazardous waste sites on the EPA's National Priorities List. ATSDR determines if
additional health studies are needed at these sites, provides health advisories
and publishes toxicological profiles on chemicals found at hazardous waste
sites.
ATSDR also maintains exposure registries of people exposed to certain
substances.
(Source:
ATSDR website
)
|
| |
|
Total suspended particulate matter found in the atmosphere as solid
particles or liquid droplets. Chemical composition of particulates
varies widely, depending on location and time of year. Airborne
particulates include: windblown dust, emissions from industrial
processes, smoke from the burning of wood and coal, and motor vehicle
or non-road engine exhausts.
|
| |
|
(in analytical chemistry)
Known amount of a homogeneous material, assumed to be taken with negligible sampling error.
Note 1: The term is usually applied to fluids.
Note 2: The term aliquot is usually used
when the fractional part is an exact divisor of the whole: the term
aliquant has been used when the fractional part is not an aexat divisor
of the whole ( e.g. a 15-ml portion is an aliquant of 100 ml).
When an aliquot is taken of a laboratory
sample or test sample or the sample is otherwise subdivided, the
samples have been called split samples.
|
| |
|
|
chemical classification of hydrocarbon groups attached to
compounds: alkyl groups have carbon atoms arranged in chains and
contain no double or triple bonds.
|
| |
|
Substance which introduces an alkyl substituent into a compound. (IUPAC)
|
| |
|
|
general term for the introduction of an alkyl group into a compound and is more specifically known as methylation, ethylation, propylation, butylation, etc., depending upon the alkyl group inserted.
|
| |
|
|
Organic mercury compounds in which the mercury is attached to an alkyl group.
Other names Alkylmercurials; Alkyl Mercury Compounds; Mercury Compounds, Alkyl; Compounds, Alkylmercury; Compounds,
|
| |
|
In discussing the fragmentation mechanism of an ion, the bond adjacent to the site of ionisation is frequently referred to as an alpha bond.
|
| |
|
|
An aluminum
compound with immune adjuvant activity. This agent adsorbs and
preciptates protein antigens in solution; the resulting precipitate
improves vaccine immunogenicity by faclitating the slow release of
antigen from the vaccine depot formed at the site of inoculation
|
| |
|
Aluminium is the worlds most common metallic element. It constitutes about 8% of the Earth's crust. It occurs in various chemical forms (species) in most rocks and soils, in vegetation and is found in most water supplies and as part of dust particles in the air. Aluminium is also present in all clays, making it an constituent of cooking vessels since earliest civilization. Evolution of human life and civilisation has developed in an aluminium rich environment.
While aluminium is abundant in the environment, the naturally occurring forms are usually stable and do not interact with the biological processes which go on in living organisms. Under acidic conditions, however, aluminium may be released from rocks and soils in a soluble form which can be absorbed by plants and animals.
|
| |
|
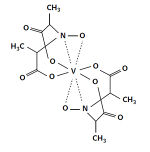 Amavadin is a vanadium-containing anion found in three species of poisonous Amanita mushrooms: A. muscaria, A. regalis, and A. velatipes. This anion, which appears as a blue solution, is an eight-coordinate vanadium complex. Amavadin is a vanadium-containing anion found in three species of poisonous Amanita mushrooms: A. muscaria, A. regalis, and A. velatipes. This anion, which appears as a blue solution, is an eight-coordinate vanadium complex.
|
| |
|
A general term describing mass spectrometry in which ionisation takes place at ambient pressure and temperature, usually in the open air. Specific types include DESI and DART.
|
| |
|
|
With more than 161,000 members, the American Chemical Society (ACS) is
the world’s largest scientific society and one of the world’s leading
sources of authoritative scientific information. A nonprofit
organization, chartered by Congress, ACS is at the forefront of the
evolving worldwide chemical enterprise and the premier professional home
for chemists, chemical engineers and related professions around the
globe. The programs and activities conducted by ACS
today are the products of a tradition of excellence in meeting member
needs that dates from the Society's founding in 1876." (Source: ACS website )
|
| |
|
|
"The American Council on Science and Health, Inc.
(ACSH) is a consumer education consortium concerned with issues related
to food, nutrition, chemicals, pharmaceuticals, lifestyle, the
environment and health. ACSH is an independent, nonprofit, tax-exempt
organization.
The nucleus of ACSH is a board of 350
physicians, scientists and policy advisors - experts in a wide variety
of fields-who review the Council's reports and participate in ACSH
seminars, press conferences, media communications and other educational
activities." (Source: ACSH website )
|
| |
|
|
A lack of crystallinity or the regular extended threedimensional-order of the atoms in a solid
|
| |
|
|
An electroanalytical technique based upon the measurement of the current
flowing through the working electrode of an electrochemical cell.
|
| |
|
|
- a compound that can be either an anion or a cation depending upon the pH of the solution in which it is.
|
| |
|
|
This can be of two kinds. White noise is random signal fluctuations
whose power spectrum contains all frequencies equally over a specified
bandwidth whereas in pink noise the frequencies diminish in a specified
fashion over a specified range.
|
| |
|
Accelerator mass spectrometry (AMS). In this specialized method, atomic ions are formed from the sample by charge stripping in a very high voltage source, usually coupled to a Van de Graff accelerator. Accelerator mass spectrometry is used for low-level analysis of 14 C isotopes in radiocarbon dating and biological tracer studies.
|
| |
|
|
An electronic device, usually based on a semiconductor chip, which samples an analogue signal, e.g. a voltage, and converts it into a digitised form. Important parameters include the number of bits that are output, the speed of conversion and the accuracy and precision.
|
| |
|
|
A statistical technique by which the observed variance can be divided
into components, such as within-run and between-run variance which make
up the total variance observed for an analytical method. Commonly used
for analysis of the data from a replication study to make estimates of
within-run and total imprecision when the data has been carefully
collected over a series of runs and days.
|
| |
|
|
The specific compound, ion, or molecule that is being determined by an analytical method.
|
| |
|
|
A measure of how far the analytical result generated with a particular method diverges from the 'true' or actual analyte concentration.
|
| |
|
Typically, the main column used in the HPLC system to separate sample components; often referred to as the "heart" of the HPLC system.The analytical column typically is in series with a short pre-column (guard) having the function of protecting the analytical column against negative effects of complex sample matrices.
|
| |
|
|
The overall, systematic procedure required to undertaken an analysis. It includes all stages of the analysis – not just the (instrumental) end determination.
|
| |
|
|
Generally defined by CLIA as an 8 hour
to 24 hour interval during which control materials must be analyzed.
According to CLSI C24, a run is “an interval (i.e., a period of time or
series of measurements) within which the accuracy and precision of the
measuring system is expected to be stable. In laboratory operations,
control samples are analyzed during each analytical run to evaluate
method performance, therefore the analytical run defines the interval
(period of time or number of specimens) between evaluations of control
results. Between quality control evaluations, events may occur causing
the measurement process to be susceptible to variations that are
important to detect.”
|
| |
|
|
The ability of an analytical
method to detect small quantities of the measured component. Numerically
characterized by determination of detection limit.
|
| |
|
|
The ability of an analytical
method to measure only the sought-for analyte or measurand. Numerically
characterized by determination of interferences and non-specific
responses to other analytes or materials.
|
| |
|
|
A negatively charged ion.
|
| |
|
|
The ability of a solid substance to adsorb anions. The anion exchange capacity of a material represents the total positive charge on the surface of the material and is generally expressed in milliequivalents per 100 grams of material (compare with cation exchange capacity).
|
| |
|
|
the ion-exchange procedure used for the separation of anions. Both resins and bonded phases are available for this mode. The tetralkylammonium group is a typical strong anion-exchange functional group. An amino group bonded on the rigid adsorbent surface would be the example of a weak anion exchanger (WAX).
|
| |
|
|
The electrode where oxidation occurs in an electrochemical cell. It is the
positive electrode in an electrolytic cell, while it is the negative electrode
in a galvanic cell. The current on the anode is considered a positive current
according to international convention; however, in electroanalytical chemistry
the anodic current is often considered negative. Contrast with cathode.
|
| |
|
|
Combined effect of two or more factors which is smaller than the solitary effect of any one of those factors. In bioassays, the term may be used when a specified effect is produced by exposure to either of two factors but not by exposure to both together.
|
| |
|
|
1. Caused by or influenced by human activities.
2. Describing a conversion factor used to calculate a dose or concentration affecting a human that has been derived from data obtained with another species (e.g., the rat). |
| |
|
|
intended to prevent fouling of underwater structures (as the bottoms of ships)
|
| |
|
|
protective coating on the body of a ship to prevent fouling by e.g. bivalves
|
| |
|
The capacity of a substance to induce the formation of antibodies or to elicit an immune response when injected into an animal.
|
| |
|
Antimony is a chemical element in the periodic table that has the symbol Sb (Latin: stibium, meaning "mark") and atomic number 51. A metalloid, antimony has four allotropic forms. The stable form of antimony is a blue-white metalloid. Yellow and black antimony are unstable non-metals. Antimony is used in flame-proofing, paints, ceramics, enamels, a wide variety of alloys, electronics, and rubber.
|
| |
|
|
Adsorbable Organic Halogens is a measurement
often used in waste water testing to indicate the overall level of
the halogens, fluorine, chlorine, bromine and iodine. This "sum
parameter" comes from a standard analytical procedure, which gives
no information on the source or nature of halogens present nor on
their toxicity. It has the advantage of being simple to measure;
alternative methods of measuring levels of individual compounds are
complex and require costly equipment.
|
| |
|
|
Atmospheric pressure chemical ionization (APCI). In this method of ionization, an aerosol of sample solution is sprayed at atmospheric pressure into a heated region in which a sharp metal pin held at high potential sustains a corona discharge. The action of the discharge on the solvent creates reagent ions that react with the neutral sample molecules to create protonated ions of the molecule. These ions then pass through a sampling aperture into the mass analyzer of the mass spectrometer.
|
| |
|
|
An "active pharmaceutical ingredient" (API) is a compound in a drug which has remedy effects on the target disorder
|
| |
|
The appearance energy (or appearance potential) is the lowest energy which must be imparted to the parent molecule to cause it to produce a particular specified ion. This energy, usually stated in electron volts, may be imparted by electron impact, by photon impact, or in other ways.
(Note: It is recommended that the term 'appearance energy' should replace the term 'appearance potential' and that the energy should be stated in SI units.)
Source: IUPAC
|
| |
|
|
One part of nitric acid and three parts of hydrochloric acid; used chiefly to dissolve metals.
|
| |
|
|
The partitioning of chemical components between various aqueous species in a solution: bare species (e.g. Ca++), ion pairs (e.g. CaCO30), and complexes (e.g. Fe(CN)63-).
|
| |
|
|
a synthetic seawater medium specifically designed for studying metal-algae interactions
|
| |
|
|
Prokaryotes lacking nucleus as bacteria but they are as different from bacteria as are humans. They represent an own evolutionary pathway. They live in extreme places with high temperatures.
|
| |
|
Detector comprising several ion/photon collection elements, arranged in a line or grid where each element is an individual detector.
|
| |
|
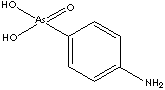 Arsanilic acid, 4-Aminophenylarsonic acid derived from orthoarsenic acid, is an arsenical antibacterial veterinary medicine used in the prevention and the treatment of swine dysentery. Arsanilic acid, 4-Aminophenylarsonic acid derived from orthoarsenic acid, is an arsenical antibacterial veterinary medicine used in the prevention and the treatment of swine dysentery.
|
| |
|
|
An arsenate (compound) is some compound that contains the arsenate ion : AsO43- . Arsenate is much like phosphate. In acid conditions we have arsenic acid, H3AsO4; in weakly acid conditions we have the dihydrogen arsenate ion, H2AsO4-; in weakly basic conditions we have hydrogen arsenate ion HAsO42-; and finally, in basic conditions, the arsenate ion AsO43-.
|
| |
|
Arsenic is a chemical element that has the symbol As and atomic number 33. Its Atomic Mass is 74.92. Its Ionic Charge is (3-). This is a notoriously poisonous metalloid that has many allotropic forms: yellow (molecular non-metallic) and several black and gray forms (metalloids) are a few that are seen. Three metalloidal forms of arsenic with different crystal structures are found free in nature (the minerals arsenic sensu strictu and the much rarer arsenolamprite and pararsenolamprite), but it is more commonly found as arsenide and arsenate compounds. Several hundred such mineral species are known. Arsenic and its compounds are used as pesticides, herbicides, insecticides and various alloys.
The most common oxidation states for arsenic are -3 (arsenides: usually alloy-like intermetallic compounds), +3 (arsenates(III) or arsenites, and most organoarsenic compounds), and +5 (arsenates(V): the most stable inorganic arsenic oxycompounds). Arsenic also bonds readily to itself, forming, for instance, As-As pairs in the red sulfide realgar and square As43- ions in the arsenide skutterudite. In the +3 oxidation state, the stereochemistry of arsenic is affected by possession of a lone pair of electrons.
|
| |
|
|
"Online focal point for the environmental health disaster in Bangladesh
and West Bengal, India, where millions of people are drinking ground
water heavily contaminated with arsenic. Site includes infobank of news
articles, scientific papers, comprehensive links to other relevant
sites, online forum, email newsletter, and local site search." (Source:
ACIC website )
|
| |
|
As2O3: an inorganic arsenic compound; a by-product of metal smelting operations.
This small-molecule arsenic compound shows antineoplastic activity. The
mechanism of action of arsenic trioxide is not completely understood.
This agent causes damage to or degradation of the promyelocytic
leukemia protein/retinoic acid receptor-alpha (PML/RARa) fusion
protein; induces apoptosis in acute promyelocytic leukemia (APL) cells
and in many other tumor cell types; promotes cell differentiation and
suppresses cell proliferation in many different tumor cell types; and
is pro-angiogenic.
|
| |
|
 Arsenicin A, As4O3(CH2)3 is a complex molecule containing four arsenic atoms in a cage-like structure. It's found in tiny quantities in the New Caledonian marine sponge Echinochalina bargibanti and is the first poly-arsenic compound to have been isolated from nature. Its role in the marine sponge remains unknown, partly because of the lack of detailed knowledge about the chemical structure. Arsenicin A, As4O3(CH2)3 is a complex molecule containing four arsenic atoms in a cage-like structure. It's found in tiny quantities in the New Caledonian marine sponge Echinochalina bargibanti and is the first poly-arsenic compound to have been isolated from nature. Its role in the marine sponge remains unknown, partly because of the lack of detailed knowledge about the chemical structure.
Empirical formula: C3H6As4O3
|
| |
|
|
An arsenide (compound) is a compound with arsenic in oxidation state -3. An arsenide ion is an arsenic atom with three extra electrons and charge -3.
|
| |
|
|
In chemistry an arsenite is a chemical compound containing an arsenic oxoanion where arsenic has oxidation state +3. Examples of arsenites include sodium arsenite which contains a polymeric linear anion, [AsO2−]n, and silver arsenite, Ag3AsO3, which contains the trigonal, AsO33− anion. The arsenite ion is sometimes called ortho-arsenite.
|
| |
|
|
The salts of the arsenous acid H3AsO3.
|
| |
|
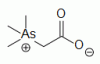 organoarsenical (CH3)3As+CH2COO-, a major arsenic containing species in marine invertebrates and fish organoarsenical (CH3)3As+CH2COO-, a major arsenic containing species in marine invertebrates and fish
|
| |
|
|
organoarsenical (CH3)3As+CH2CH2OH, a major arsenic containing species in marine invertebrates and fish
|
| |
|
Natural arsenolipids are analogues of neutral lipids, like
monoglycerides, glycolipids, phospho- and also phosphonolipids. They
have been found in microorganisms, fungi, plants, lichens, in marine
mollusks, sponges, other invertebrates, and in fish tissues.
Arsenolipids are thought to be end products of arsenate detoxification
processes, involving reduction and oxidative methylation and
adenosylation.
Source:
Valery M. Dembitsky, Dmitrii O. Levitsky, Arsenolipids, Progress in Lipid Research, 43/5 (2004) 403-448.
|
| |
|
FeAsS, Iron Arsenide Sulfide, an inorganic arsenic compound. This mineral is a major ore of arsenic.
For more information on the mineral Arsenopyrite see: Mineral Gallery: The Mineral Arsenopyrite
|
| |
|
|
Arsenoribofuranosides are organoarsenicals containing a pentose moiety as part of their molecular structure (see figure), which explains why they are commonly referred to arsenosugars.
Arsenosugars are major arsenic compounds in algae but are reported to be present in significant concentrations in other marine organisms such as bivalves. This class of compounds has been raising growing concern since recent reports indicating the possibility of metabolizing arsenosugars to the carcinogenic dimethylarsinic acid (DMAA) by the human body.
|
| |
|
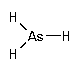 High-purity arsine is exclusively used in semiconductor manufacturing. The arsenic atom is an n-type dopant for epitaxial silicon. Arsenic is introduced in the silicon wafer by diffusion or implantation techniques. The presence of traces of arsine in the environment has been observed in gas purged from natural waters and in hot springs.
CAS Number : 7784-42-1
Other names: Arsenic hydride; Arsenic trihydride; Arsenous hydride; Hydrogen arsenide; Arsenic hydrid
|
| |
|
|
chemical classification of hydrocarbon groups attached to compounds: aryl groups have carbon atoms arranged in aromatic rings.
|
| |
|
|
Organoarsenic compounds in which arsenic is bound onto phenyl.
The structures of the arylarsenicals that are approved as animal-food additives are shown below.
4-Hydroxy-3-nitrophenylarsonic acid (3-NHPAA) and p-arsanilic acid (p-ASA) are approved for poultry and swine. 4-Nitrophenylarsonic acid (4-NPAA) and p-ureidophenylarsonic acid (p-UPAA) are approved only for controlling blackhead disease in turkeys.
There are some compounds, such as Clark-1, Clark-2 and Adamsite, which have been developed during the first World War as warfare chemicals. Some of their degradation products (e.g. diphenyl arsinic acid) can also been found in the environment close to former production or dump sites.
|
| |
|
The decomposition, prior to analysis, of organic matrix constituents of the sample. The most common ashing techniques are acid or alkali dissolution: alkaline fusion; and oxidation using either low-temperature oxygen plasma or muffle furnace.
  
|
| |
|
|
Sometimes used to mean a test or test result, but other times as a synonym for the analytical method.
|
| |
|
Asymmetrical flow FFF (AFFFF or AF4) is a type of flow FFF using only one permeable wall which serves as accumulation wall. Experiments show that the asymmetrical design separates faster and more efficient than the symmetrical channel.
AFFFF is certainly one of the most universal FFF techniques. It features a very broad (105) separation range from 1 nm to 100 µm . And its application range covers both simple and complex macromaterials of biological, pharmaceutical, industrial and environmental relevance such as proteins, polymers or nanoparticles.
|
| |
|
|
A term used to describe instrumentation that chemically quantifies or qualifies materials in near real-time (as they are produced) near a production line. Generally, a sample aliquot is taken from the production line and moved to an analysis station for manual sample presentation to a nearby instrument.
|
| |
|
Process that transfers a chemical from the atmosphere to the Earth’s surface (land, water, or vegetation) by either dry impingement or by transport in rain or snow.
See also dry deposition, wet deposition.
|
| |
|
Atmospheric pressure ionization (API) is any process in which ions are formed from atoms or molecules at atmospheric pressue, such as in ESI, APCI, APPI and MALDI.
|
| |
|
A form of API in which the analyte is sprayed into the source, as in ESI and APCI sources, in a solvent frequently containing a dopant molecule such as toluene or acetone. The spray is irradiated by a powerful UV source that forms excited species which undergo secondary ion/radical- molecule reactions with the analytes to cause ionisation. It is claimed to be the method of choice for detection of non-polar molecules in HPLC eluates. Protonated or deprotonated molecules or radical molecular ions can be formed, according to the mechanisms and thermodynamics involved.
|
| |
|
|
A device designed to produce a collimated beam of energetic atoms from a heated source. In MS, this is the prime source for the formation of ions in a FAB source. Most SIMS and FAB sources use atom as well as ion guns, commonly with xenon atoms or caesium ions.
|
| |
|
|
A spectroscopic technique that responds to free atoms in the gaseous phase. The diminuation in the intensity of analyte specific monochromatic radiation which is directed through the atom cloud is related to the quantity of analyte atoms present in that cloud.
|
| |
|
|
A group of spectroscopic techniques that respond to free atoms or ions
in the gasepous phase. The intensity of the energy emitted as the
analyte atoms/ions spontaneously return from an excited state(s) to
their lowest energy state is related to the quantity of that analyte.
|
| |
|
|
In atomic fluorescence spectrometry (AFS), ground state atoms created chemically or thermally in a hydrogen flame or quartz tube furnace, are excited by a beam of light. In difference to AAS measuring the absorbed light, the emission resulting from the decay of the excited state is measured. AFS is a very specific element detection technique but requires a high output, high stability light source.
|
| |
|
|
The number of protons in an atomic nucleus
|
| |
|
The analytical principle of atomic spectroscopy is
based on the property of atoms of emitting or absorbing element-specific
electromagnetic radiation under certain physical conditions. To this
end, it is necessary to release the elements to be investigated in a
sample from their compounds, generally by the input of energy, and to
make them available as free particles.
|
| |
|
The nominal atomic weight of an isotope is given by the sum of the
number of neutrons and protons in each nucleus. The exact atomic weight
differs fractionally from that whole number, because neutrons are
slightly heavier than protons and the mass of the nucleus is also
affected by the binding energy.
|
| |
|
|
Conversion of molecular or condensed forms of an analyte into free atoms.
|
| |
|
|
Reduces the amplitude of the electrical signal generated by the detector, with little or no distortion. Usually carried out by applying a control voltage to extend the dynamic range of the detector. Also refer to extended dynamic range (EDR).
|
| |
|
|
An electron beam technique applied for near-surface elements identification often used in thin film science. When the sample surface is bombarded with a beamn of electrons, surface electrons are emitted. The energy, intensity and location of these emitted electrons are analyzed to determine the elements present.
|
| |
|
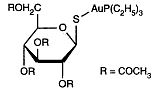 Auranofin is a metallodrug used in treating inflammatory arthritis. Exactly how such gold compounds work is not well understood. In patients with inflammatory arthritis, such as adult and juvenile rheumatoid arthritis, gold salts can decrease the inflammation of the joint lining. Auranofin is a metallodrug used in treating inflammatory arthritis. Exactly how such gold compounds work is not well understood. In patients with inflammatory arthritis, such as adult and juvenile rheumatoid arthritis, gold salts can decrease the inflammation of the joint lining.
|
| |
|
|
Autism is a brain disorder that affects a person’s ability to communicate,
relate to others, and interact with his or her surroundings. Autism is usually
diagnosed between the ages of two and three based on observation of behaviour. There is no cure for autism, except
early diagnosis and treatment, which may help to ease the symptoms.
|
| |
|
|
Self-catalyzed oxidation reaction that occurs spontaneously in an aerobic environment.
|
| |
|
|
Average Deviation refers to the summation of the deviation divided by the number of results.
|
| |
|
|
The average mass of an ion of a known empirical formula is calculated by summing the relative average atomic mass of each atom present. For example, carbon has an average atomic mass of 12.01115 Da, hydrogen is 1.00797 Da, and so on. The average mass of the molecular ion of a chemical compound is also the mass that appears on the bottle. However, in a stick representation of a mass spectrum, there is no ion signal at the average mass. Instead, signals appear for ions of various isotopic compositions. The average mass corresponds to the center of the centroid signal recorded for higher mass ions at lower instrumental resolutions.
|
| |
|
|
An ICP-OES system in which the plasma torch is positioned end-on to the optical system as opposed to the conventional lateral (radial) configuration. It is generally accepted that viewing the end of the plasma improves emission intensity by a factor of approximately 5- to 10-fold but also enhances interference effects.
|
| |
|
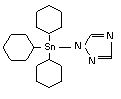 Azocyclotin is an organotin compound used as an insecticide. The product is a contact acaricide. Azocyclotin is an organotin compound used as an insecticide. The product is a contact acaricide.
| IUPAC: |
tri(cyclohexyl)-1H-1,2,4-triazol-1-yltin |
CAS:
|
1-(tricyclohexylstannyl)-1H-1,2,4-triazole |
| Reg. No.: |
41083-11-8 |
Formula:
|
C20H35N3Sn |
|
| |
|