Speciation analysis is aiming to define and quantify the distribution of a target element between the different species in which it occurs. While it is well accepted that information provided by such speciation analysis is mandatory for a complete understanding of processes in biological systems or the environment, the lack of proven quality of such data is seriously limiting its value.
Unfortunately, concepts for quality control and quality assurance widely implemented in the field of trace element analysis are not often applied to speciation analysis. The higher complexity of speciation analysis in comparison to total element analysis is often used as an excuse for the lacking validation. However, apart from the higher complexicity, speciation analysis shares most problems also known in the field of trace element analysis and therefore can also gain from such quality concepts.
The most problematic steps of speciation analysis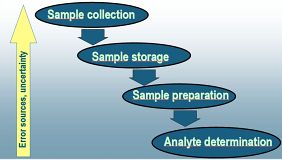
While the final steps of the analytical procedure including the analytical measurement itself receive the greatest attention, actually the first steps of analysis including sampling, sample storage and sample preparation are the most problematic ones. While this is true not only for speciation analysis but for all chemical analysis procedures, speciation analysis has additional problems in these steps. The major problem of speciation analysis in the course of these steps is to maintain the original distribution of species (speciation) through all the steps until the final step of determination and quantitation. The ideal solution to this problem would be
in situ analysis using sensors. However, very few sensors provide the necessary selectivity and sensitivity required for trace element speciation analysis.
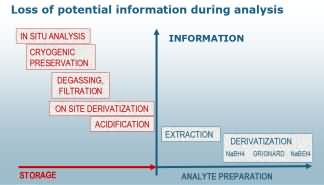
A specific problem of speciation analysis is that original information (molecular integrity) of the species is partially being lost as the sample preparation progresses. Therefore, the best sample preparation for speciation analysis is no preparation at all.
Unfortunately, only few techniques such as EXAFS/XANES provide species data from solid samples. For all other techniques the reduction of the sample preparation to the absolutely necessary number of steps remains the appropriate strategy.
Species transformation during analysisMost often the selectivity and sensitivity required for trace elemental speciation analysis calls for the use of hyphenated techniques combing the separation power of a chromatographic or equivalent separation technique with the detection power of the most sensitive atomic spectroscopic techniques (e.g. ICP-MS, MIP-AES, AFS). These techniques call for liquid samples and therefore sample preparation can seldom be avoided. In these cases obtaining meaningful results requires careful validation of each of the steps of the sample preparation in order to avoid artefacts by species transformation or losses during analysis.
- Sampling: Changes of pH, pE should be avoided; microbial activity should be stopped, stability of species should be verified
- Preservation: Stability of species during sample storage should be verified; absence of analyte losses by sorption to container walls or volatilization should be proven
- Extraction: Excessive energy input (e.g. heating etc.) and harsh conditions (oxidants etc.) must be avoided; stability of species during extraction must be verified; extraction yield should be monitored
- Derivatization: Absence of species transformation during derivatization should be proven; quantitative derivatization yield should be proven
- Separation: absence of species transformation during separation should be proven; column yield should be checked by mass balance with total element concentration; appropriate separation power must be verified;
- Detection/Identification: Unambiguous identification of species (e.g. via molecular mass and retention time) should be verified; "identification" of species in the void volume of chromatograms should be avoided;
- Calibration/Quantification: calibration should be done by using reference standards or reference materials; absence of interferences should be proven; factors influencing the result should be assessed systematically; uncertainty of the result should be estimated based on scientific understanding of the theoretical principles of the method and practical experience; results should be compared to those achieved with other methods
Meaningful validation for speciation analysisBy carefully looking at the above list of necessary tests it becomes clear that
total element analysis should be an integral part of speciation analysis. Without knowing the part of the analyte that was determined, the whole speciation analysis becomes very questionable. A meaningful quality assurance and quality control (QA/QC) frequently includes analysis of a Certified Reference Material (CRM), comparison of results from independent techniques, or analysis of a proficiency test sample. The validation should be as extensive as is necessary to meet the needs of the given application (fit-for-purpose) and the range and accuracy of the values obtainable are all relevant figures of merit (e.g. the uncertainty of the results, detection limit, selectivity of the method, linearity, repeatability and/or reproducibility, robustness against external influences and/or cross-sensitivity against interference
from the matrix of the sample or test object), as assessed for the intended use.
While most peer reviewers expect to see such corroboration of results and many journal editors are at least aware of the importance of doing so for total element analysis, this is not yet the case for speciation analysis. While it is true that the number of CRMs for speciation analysis is rather limited (see our list), many of the above mentioned tests improving the credibility of results can be applied even when no appropriate CRM is available. An alternative way is the use of a primary method, such as species-specific isotope dilution analysis (SIDMS). SIDMS allows corrections for extraction yields, species inter-conversions and quantitation based on an ideal internal standard when suitably
characterised enriched species are available.
Interestingly, journal editors have started to recognize the lack of quality related strategies implemented in submitted papers. As one of the first journals
Environmental Chemistry has now published rules for
reporting speciation data as a part of their
notice to authors. We encourage all editors but also reviewers to request the performance of such procedures especially when authors suggest new speciation analytical methods.
 Publications discussing reliability of speciation analysis (general discussion)
Publications discussing reliability of speciation analysis (general discussion)
 Heidi Goenaga Infante
Heidi Goenaga Infante,
Quality Control in Speciation Analysis Using HPLC with ICP-MS and ESI-MS/MS: Focus on Quantification Strategies Using Isotope Dilution Analysis. In: Bernhard Michalke (ed.), Metallomics: Analytical Techniques and Speciation Methods, Wiley-VCH, Weinheim, 2016, 69-82.
DOI: 10.1002/9783527694907.ch3
 Ralph E. Sturgeon
Ralph E. Sturgeon,
Kevin A. Francesconi,
Enhancing reliability of elemental speciation results - quo vadis?, Environ. Chem., 6/4 (2009) 294-297.
DOI: 10.1071/EN09074
Graeme E. Batley,
Kevin A. Francesconi,
William A. Maher,
The role of speciation in environmental chemistry and the case for quality criteria, Environ. Chem., 6/4 (2009) 273-274.
doi: 10.1071/EN09093  H. Goenaga-Infante
H. Goenaga-Infante,
R. Sturgeon, J. Turner, R. Hearn, M. Sargent, P. Maxwell, L. Yang, A. Barzev, et al.,
Total selenium and selenomethionine in pharmaceutical yeast tablets: assessment of the state of the art of measurement capabilities through international intercomparison CCQM-P86, Anal. Bioanal. Chem., 390 (2008) 629.
doi:10.1007/S00216-007-1654-8 J. Meija
J. Meija, Z. Mester,
Paradigms in isotope dilution mass spectrometry for elemental speciation analysis, Anal. Chim. Acta, 607 (2008) 115.
doi:10.1016/J.ACA.2007.11.050 Kevin A. Francesconi
Kevin A. Francesconi,
Michael Sperling,
Speciation analysis with HPLC-mass spectrometry: time to take stock, Analyst (London), 130/7 (2005) 998-1001.
DOI: 10.1039/b504485p P. Quevauviller
P. Quevauviller,
Traceability of environmental chemical analyses: can theory match practice?, Anal. Bioanal. Chem., 382 (2005) 1800.
doi:10.1007/S00216-005-3384-0 Ph. Quevauviller
Ph. Quevauviller,
O. F. X. Donard,
Ensuring quality in long term environmental monitoring for chemical speciation, J. Environ. Monit., 1/5 (1999) 503-513.
DOI: 10.1039/a904086b Bernhard Michalke
Bernhard Michalke,
Quality control and reference materials in speciation analysis, Fresenius J. Anal. Chem., 363/5-6 (1999) 439-445.
doi: 10.1007/s002160051219 Philippe Quevauviller
Philippe Quevauviller,
Improvement of quality control of speciation analysis using hyphenated techniques - A decade of progress within the European Community, J. Chromatogr. A, 750 (1996) 25-33.
DOI: 10.1016/0021-9673(96)00293-2 Publications discussing problems of speciation analysis
Publications discussing problems of speciation analysis J. Meija
J. Meija, L. Ouerdane, Z. Mester,
Isotope scrambling and error magnification in multiple-spiking isotope dilution, Anal. Bioanal. Chem., 394 (2009) 199.
doi:10.1007/S00216-009-2619-X

H. Emons,
Artefacts and facts about metal(loid)s and their species from analytical
procedures in environmental biomonitoring, Trends Anal. Chem. (Pers. Ed.), 21/6-7 (2002) 401-411.
doi:10.1016/S0165-9936(02)00606-4
Gemma Rauret, R. Rubio,
Sources of error in speciation analysis, Quim. Anal. (Barcelona), 16/S.1 (1997) S119-S130.
 H. Hintelmann
H. Hintelmann, R. Falter, G. Ilgen, R.D. Evans,
Determination of artifactual formation of monomethylmercury (CH3Hg+) in environmental samples using stable Hg²+ isotopes with ICP-MS detection: Calculation of contents applying species specific isotope addition, Fresenius J. Anal. Chem., 358/3 (1997) 363-370.
doi: 10.1007/s002160050431
Philip Ekow Gardiner,
Factors that Influence the Distribution of Trace Element-Containing Chemical Species in Living Systems before and after Sample Collection, J. Trace Elem. Electrol. Health Dis., 7 (1993) 1-8.
 Studies discussing validation of speciation methods
Studies discussing validation of speciation methods

S. J. Nagourney, S. A. Wilson and S. E. Long,
Using reference materials to improve the quality of data generated by USEPA analytical methods, Environ. Sci.: Processes Impacts, 2016, 18, 1477.
DOI: 10.1039/c6em00438

Sébastien Sannac,
Florence Pannier, Caroline Oster, Guillaume Labarraque, Paola Fisicaro,
Martine Potin-Gautier,
Validation of a reference measurement procedure for the assessment of selenomethionine in nutritional supplements, J. Anal. At. Spectrom., 24/2 (2009) 237-241.
DOI: 10.1039/b808663j
C.S.J. Wolff Briche,
R. Wahlen,
R. E. Sturgeon,
CCQM-P43: Tributyltin and dibutyltin in sediment, Metrologia, 43(Tech. Suppl.) (2006) 08002.
DOI:10.1088/0026-1394/43/1A/08002
 Robert Clough
Robert Clough, Simon T. Belt, Ben Fairman, Tim Catterick, E. Hywel Evans,
Uncertainty contributions to single and double isotope dilution mass spectrometry with HPLC-CV-MC-ICP-MS for the determination of methylmercury in fish tissue, J. Anal. At. Spectrom., 20/10 (2005) 1072.
DOI: 10.1039/b502670a G.M. Mizanur Rahman
G.M. Mizanur Rahman,
H.M. Skip Kingston, John C. Kern, Sara W. Hartwell, Raymond F. Anderson, Shen-yi Yang,
Inter-laboratory validation of EPA method 3200 for mercury speciation analysis using prepared soil reference materials, Appl. Organomet. Chem., 19/3 (2005) 301-307.
DOI: 10.1002/aoc.816 Nicolas S. Bloom
Nicolas S. Bloom, A.K. Grout, E.M. Prestbo,
Development and complete validation of a method for the determination of dimethyl mercury in air and other media, Anal. Chim. Acta, 546/1 (2005) 92-101.
DOI:10.1016/j.aca.2005.04.087
Lars Lambertsson,
Erik Björn,
Validation of a simplified field-adapted procedure for routine determinations of methyl mercury at trace levels in natural water samples using species-specific isotope dilution mass spectrometry, Anal. Bioanal. Chem., 380/7-8 (2004) 871-875.
DOI: 10.1007/s00216-004-2863-z Robert Clough
Robert Clough, Simon T. Belt,
E. Hywel Evans, Ben Fairman, Tim Catterick,
Uncertainty contributions to species specific isotope dilution analysis. Part 2. Determination of methylmercury by HPLC coupled with quadrupole and multicollector ICP-MS, J. Anal. At. Spectrom., 18/9 (2003) 1039-1046.
DOI: 10.1039/b305454n Robert Clough
Robert Clough, Simon T. Belt,
E. Hywel Evans, Pete Sutton, Ben Fairman, Tim Catterick,
Uncertainty contributions to species specific isotope dilution analysis. Part 1. Characterisation and stability of 199Hg and 13C isotopically enriched methylmercury by 1H NMR, J. Anal. At. Spectrom., 18/9 (2003) 1033-1038.
DOI: 10.1039/b302880c
Sandrine Aguerre,
Christophe Pécheyran,
Gaëtane Lespes,
Validation, using a chemometric approach, of gas chromatography-inductively coupled plasma-atomic emission spectrometry (GC-ICP-AES) for organotin determination, Anal. Bioanal. Chem., 376/2 (2003) 226-235.
DOI 10.1007/s00216-003-1898-x Bernhard Michalke
Bernhard Michalke, P. Schramel,
Selenium speciation in human milk with special respect to quality control, Biol. Trace Elem. Res., 59 (1997) 45-56.
DOI: 10.1007/BF02783229
 Related EVISA Resources
Related EVISA Resources Brief summary: Certified Reference Materials for Speciation Analysis
Brief summary: Certified Reference Materials for Speciation Analysis Brief summary: What is the use for total element concentrations ?
Brief summary: What is the use for total element concentrations ? Brief summary: Sample preservation for speciation analysis - General recommendations
Brief summary: Sample preservation for speciation analysis - General recommendations Materials Database
Materials Database Link page: All about QA/QC
Link page: All about QA/QC Link page: All about CRMs
Link page: All about CRMs Information related to quality management, assurance and control
Information related to quality management, assurance and control
 CTDEP: Quality Assurance and Quality Control (QA/QC) Guidance
CTDEP: Quality Assurance and Quality Control (QA/QC) Guidance USEPA: Clean Water Act Analytical Methods - Quality Assurance and Quality Control Requirements in Methods Not Published by EPA
USEPA: Clean Water Act Analytical Methods - Quality Assurance and Quality Control Requirements in Methods Not Published by EPA NIST (2002). Statistical Methods of Uncertainty Analysis
NIST (2002). Statistical Methods of Uncertainty Analysis EURACHEM/Citac Guide: Quantifying Uncertainty in Analytical Measurement, Third Edition
EURACHEM/Citac Guide: Quantifying Uncertainty in Analytical Measurement, Third Edition EURACHEM/CITAC Guide: Measurement uncertainty arising from sampling: A guide to methods and approaches, 2nd edition 2019
EURACHEM/CITAC Guide: Measurement uncertainty arising from sampling: A guide to methods and approaches, 2nd edition 2019 EURACHEM Guide: The selection and use of reference materials
EURACHEM Guide: The selection and use of reference materials EURACHEM/CITAC Guide: Assessment of performance and uncertainty in qualitative testing
EURACHEM/CITAC Guide: Assessment of performance and uncertainty in qualitative testing EURACHEM/CITAC guide: Metrological Traceability in Chemical Measurement
EURACHEM/CITAC guide: Metrological Traceability in Chemical Measurement Further chapters on techniques and methodology for speciation analysis:
Further chapters on techniques and methodology for speciation analysis:
 Chapter 1:
Tools for elemental speciation
Chapter 1:
Tools for elemental speciation Chapter 2: ICP-MS - A versatile detection system for speciation analysis
Chapter 2: ICP-MS - A versatile detection system for speciation analysis Chapter 3: LC-ICP-MS - The most often used hyphenated system for speciation analysis
Chapter 3: LC-ICP-MS - The most often used hyphenated system for speciation analysis Chapter 4: GC-ICP-MS- A very sensitive hyphenated system for speciation analysis
Chapter 4: GC-ICP-MS- A very sensitive hyphenated system for speciation analysis Chapter 5: CE-ICP-MS for speciation analysis
Chapter 5: CE-ICP-MS for speciation analysis Chapter 6: ESI-MS: The tool for the identification of species
Chapter 6: ESI-MS: The tool for the identification of species Chapter 7: Speciation Analysis - Striving for Quality
Chapter 7: Speciation Analysis - Striving for Quality  Chapter 8: Atomic Fluorescence Spectrometry as a Detection System for Speciation Analysis
Chapter 8: Atomic Fluorescence Spectrometry as a Detection System for Speciation Analysis  Chapter 9: Gas chromatography for the separation of elemental species
Chapter 9: Gas chromatography for the separation of elemental species  Chapter 10: Plasma source detection techniques for gas chromatography
Chapter 10: Plasma source detection techniques for gas chromatography Chapter 11: Fractionation as a first step towards speciation analysis
Chapter 11: Fractionation as a first step towards speciation analysis  Chapter 12: Flow-injection inductively coupled plasma mass spectrometry for speciation analysis
Chapter 12: Flow-injection inductively coupled plasma mass spectrometry for speciation analysis  Chapter
13: Gel electrophoresis combined with laser ablation inductively
coupled plasma mass spectrometry for speciation analysis
Chapter
13: Gel electrophoresis combined with laser ablation inductively
coupled plasma mass spectrometry for speciation analysis  Chapter 14: Non-chromatographic separation techniques for speciation analysis
Chapter 14: Non-chromatographic separation techniques for speciation analysis Related EVISA News (newest first)
Related EVISA News (newest first)
last time modified: June 5, 2025