Electrochemical techniques provide valuable tools for speciation analysis due to their high sensitivity, selectivity, and ability to work in complex matrices.
Speciation analysis is typically accomplished by combining extraction and separation techniques with various detection methods. However, a challenge arises when the analyte is separated from its natural environment, leading to changes in chemical equilibrium and potential alterations in chemical species during extraction and separation steps. Ideally, analytical techniques enabling the direct detection and quantification of species are preferred. Trace metal species are generally categorized as either particulate or dissolved forms. For the direct analysis of solid species, only X-ray-based techniques can be applied.
While the direct measurement of dissolved trace species using "sensors" was a hopeful prospect for a considerable period, it fell short of expectations for various reasons, including lack of selectivity, sensitivity, and long-term stability. In contrast, electrochemical techniques prove highly suitable for the direct measurement of dissolved metal species with minimal or no sample preparation. The instrumentation required is minimal, usually limited to a potentiostat, a computer, a reference electrode, and a working electrode (see Figure).
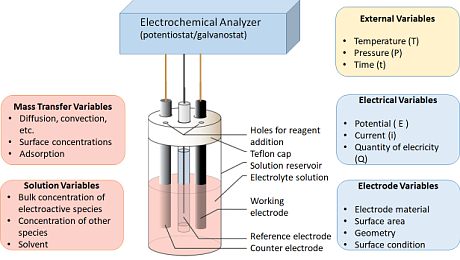
Figure: Principal setup of electrochemical analysis
These techniques facilitate on-site or in situ measurements, minimizing the risk of speciation change or sample contamination. Additionally, the design of electrodes with very small dimensions allows for reduced sample consumption, making electrochemical techniques particularly suitable for continuous, automatic, and real-time analysis.
Two main types of electrochemical analysis are potentiometry with ion-selective electrodes (ISEs) and voltammetry. Potentiometry involves the direct measurement of the electrode potential once equilibrium is reached between the electrode membrane and the solution, with the potential directly related to the ion concentration. Voltammetry, on the other hand, varies the working electrode potential and measures the resulting current, allowing for the identification of metals based on the potential at which the current flows.
Voltammetry is especially valuable for measuring trace metal species at environmental levels when combined with preconcentration by electrodeposition, forming the technique of stripping voltammetry. This includes anodic stripping voltammetry (ASV), cathodic stripping voltammetry (CSV), and adsorptive stripping voltammetry. These methods involve accumulating electroactive analytes on the working electrode's surface during electrodeposition, followed by oxidation (ASV) or reduction (CSV) back into the solution during the stripping step. The enrichment effect can lead to up to four orders of magnitude enhancement. Stripping responses, in the form of voltammograms, are utilized for qualitative and quantitative analyses, offering sub-ppb limits of detection.
In natural water, metal ions often exist in the form of complexes, complicating their identification. ASV, however, can distinguish between electrically active (hydrated metal ions or labile coordination ions) and electrically inactive (organic complexes and colloidal forms) metal species. The total concentration of metals within these complexes, referred to as labile metals, is crucial for assessing bioavailability and toxicity. Despite the term "labile state" lacking a precise definition, it commonly includes hydrated metal ions and weakly organically bound metal complexes, which are essential considerations in eco-toxicology models such as the Free Ion Activity Model (FIAM) and the Biotic Ligand Model (BLM).
As these methods measure fractions of complexes rather than single species, they are termed fractionation methods according to IUPAC. Since electrochemical techniques can thus detect complexes with various strengths and labilities by tuning the experimental parameters, it is recommended that detailed experimental conditions should be stated when using the word "labile".
Michael Sperling
 Related publications reviewing the topic (newest first)
Related publications reviewing the topic (newest first)

Michel Meyer, Demetrio Milea,
Emerging Analytical Techniques for Chemical Speciation Studies. Part 1: Electrochemical and Migration Methods, World Scientific, Singapore, 2025.
DOI: 10.1142/13537

Vasiliki Keramari, Sophia Karastogianni, Stella Girousi,
New Prospects in the Electroanalysis of Heavy Metal Ions(Cd, Pb, Zn, Cu): Development and Application of Novel Electrode Surfaces, Methods Protoc., 6 (2023) 60.
DOI: 10.3390/mps6040060
Diana Amorello, Santino Orecchio, Salvatore Barreca, Silvia Orecchio,
Voltammetry for Monitoring Platinum, Palladium and Rhodium in Environmental and Food Matrices, ChemistrySelect, 8/18 (2023) e202300200.
DOI: 10.1002/slct.202300200

Lucía López-Solis , Josep Galceran , Jaume Puy, Encarna Companys,
Absence of Gradients and Nernstian Equilibrium Stripping (AGNES): An Electroanalytical Technique for Chemical Speciation: A Tutorial Review, Chemosensors, 10 (2022) 351.
DOI: 10.3390/chemosensors10090351
Jaroslav Filip, Štěpán Vinter, Erika Čechová, Jitka Sotolářová,
Materials interacting with inorganic selenium from the perspective of electrochemical sensing, Analyst, 146 (2021) 6394.
DOI: 10.1039/d1an00677k
P. Sánchez-Marín,
A review of chemical speciation techniques used for predicting dissolved copper bioavailability in seawater, Environ. Chem., 17/7 (2020) 469-478.
DOI: 10.1071/EN19266

Nazha Hilali, Hasna Mohammadi, Aziz Amine, Nadia Zine, Abdelhamid Errachid,
Recent Advances in Electrochemical Monitoring of Chromium, Sensors, 20 (2020) 5153;
DOI: 10.3390/s20185153
James A. Cox, Iwona A. Rutkowska, Pawel J. Kulesza,
Critical Review—Electrocatalytic Sensors for Arsenic Oxo Species, J. Electrochem. Soc., 167 (2020) 037565
DOI: 10.1149/1945-7111/ab697d
Alexandra J. Borrill, Nicole E. Reily, Julie V. Macpherson,
Addressing the practicalities of anodic stripping voltammetry for heavy metal detection: a tutorial review, Analyst, 144 (2019) 6834.
DOI: 10.1039/c9an01437c
Pooja Devi, Rishabh Jain, Anupma Thakur, Manish Kumar, Nitin K. Labhsetwar, Manoj Nayak, Praveen Kumar,
A systematic review and meta-analysis of voltammetric and optical techniques for inorganic selenium determination in water, Trends Anal. Chem., 95 (2017) 69-85.
DOI: 10.1016/j.trac.2017.07.012

E. Companys, J. Galceran, J.P. Pinheiro, J. Puy, P. Salaün,
A review on electrochemical methods for trace metal speciation in environmental media, Curr. Opin. Electrochem., 3 (2017) 144–162.
DOI: 10.1016/j.coelec.2017.09.007
Luis M. Laglera, Damiano Monticelli,
Iron detection and speciation in natural waters by electrochemical techniques: A critical review, Current Opinion in Electrochem., 3 (2017) 123–129.
DOI: 10.1016/j.coelec.2017.07.007
Alex L. Suherman, Eden E.L. Tanner, Richard G. Compton,
Recent developments in inorganic Hg2ţ detection by voltammetry, Trends Anal. Chem., 94 (2017) 161-172.
DOI: 10.1016/j.trac.2017.07.020
Nikolaos Kallithrakas-Kontos, Spyros Foteinis,
Recent Advances in the Analysis of Mercury in Water - Review, Curr. Anal. Chem., 12 (2016) 22-36.
DOI: 10.2174/157341101201151007120324

Svetlana Antonova, Elza Zakharova,
Inorganic arsenic speciation by electroanalysis. From laboratory to field conditions: A mini-review, Electrochemistry Communications 70 (2016) 33–38.
DOI: 10.1016/j.elecom.2016.06.011
Dario Omanović, Cédric Garnier, Kristoff Gibbon–Walsh, Ivanka Pižeta,
Electroanalysis in environmental monitoring: Tracking trace metals—A mini review, Electrochem. Commun., 61 (2015) 78–83.
DOI: 10.1016/j.elecom.2015.10.007
Rakesh R. Chillawar, Kiran Kumar Tadi, and Ramani V. Motghare,
Voltammetric Techniques at Chemically Modified Electrodes, J. Anal. Chem., 70/4 (2015) 399–418.
DOI: 10.1134/S1061934815040152
Kathryn E. Toghill, Min Lu, Richard G. Compton, E
lectroanalytical Determination of Antimony, Int. J. Electrochem., 6/8 (2011) 3057-3076.
DOI: 10.1016/S1452-3981(23)18236-8
Douglas E. Mays, Abul Hussam,
Voltammetric methods for determination and speciation of inorganic arsenic in the environment—A review, Anal. Chim. Acta, 646/1–2 (2009) 6-16.
DOI: 10.1016/j.aca.2009.05.006 
Andrzej Bobrowski, Agnieszka Królicka, Jerzy Zarebski,
Characteristics of Voltammetric Determination and Speciation of Chromium – A Review, Electroanalysis 2009, 21, No. 13, 1449 – 1458.
DOI: 10.1002/elan.200904582

G.E. Batley, S.C. Apte, J.L. Stauber,
Speciation and bioavailability of trace metals in water: Progress since 1982, Austr. J. Chem., 57/10 (2004) 903-919.
DOI: 10.1071/CH04095 
J. Labuda, M. Vanicková, M. Bucková, E. Korgová,
Development in Voltammetric Analysis with Chemically Modified Electrodes and Biosensors, Chem. Papers, 54/2 (2000) 95—103.
last time modified: October 9, 2025