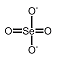
Selenates are analogous to sulfates and have similar chemistry. Unlike sulfate, selenate is a somewhat good oxidizer; it can be reduced to selenite or selenium. In strongly acid conditions, hydrogen selenate ion, HSeO
4-, is formed; the selenic acid, H
2SeO
4, is very strong