|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
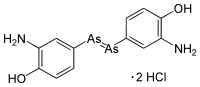 Arsphenamine, also known as Salvarsan and 606, is a drug containing arsenic that was used to treat syphilis and trypanosomiasis. The organoarsenic compound was the first modern chemotherapeutic agent. Arsphenamine was marketed under the trade name Salvarsan in 1910. It was also called 606, because it was the 606th compound synthesized for testing. Salvarsan was the first organic anti-syphillitic, and a great improvement over the inorganic mercury compounds that had been used previously. A more soluble (but slightly less effective) arsenical compound, Neosalvarsan, (neoarsphenamine), became available in 1912. These arsenical compounds came with considerable risk of side effects, and they were supplanted as treatments for syphilis in the 1940s by penicillin. Arsphenamine, also known as Salvarsan and 606, is a drug containing arsenic that was used to treat syphilis and trypanosomiasis. The organoarsenic compound was the first modern chemotherapeutic agent. Arsphenamine was marketed under the trade name Salvarsan in 1910. It was also called 606, because it was the 606th compound synthesized for testing. Salvarsan was the first organic anti-syphillitic, and a great improvement over the inorganic mercury compounds that had been used previously. A more soluble (but slightly less effective) arsenical compound, Neosalvarsan, (neoarsphenamine), became available in 1912. These arsenical compounds came with considerable risk of side effects, and they were supplanted as treatments for syphilis in the 1940s by penicillin.
Source: Wikipedia
|
| |
|
|
A portion or piece of a whole. A selected subset of a population or subset of whatever is being studied. For example, in a study of people the sample is a number of people chosen from a larger population [see population]. An environmental sample (for example, a small amount of soil or water) might be collected to measure contamination in the environment at a specific location.
|
| |
|
|
- a collective term referring to all of the sample constituents other than the analyte(s).
|
| |
|
|
That part of the total error (the estimate from a sample minus the population value) associated with using only a fraction of the population and extrapolating to the whole, as distinct from analytical or test error.
Note: Sampling error arises from a lack of homogeneity in the parent population.
|
| |
|
|
An orally administered third generation platinum compound with potential antineoplastic activity. Satraplatin
forms highly reactive, charged, platinum complexes which bind to
nucleophilic groups in DNA, inducing intrastrand and interstrand DNA
cross-links, as well as DNA-protein cross-links. These cross-links
result in cell growth inhibition and apoptosis.
|
| |
|
A solution which has the same concentration of a solute as one that is in equilibrium with undissolved solute at specified values of temperature and pressure.
Source: IUPAC
|
| |
|
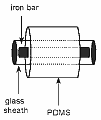 Stir bar sorptive extraction (SBSE) is a solid phase extraction (SPE) method in which the sorbent is a coating of a solid magnetical stir bar. SBSE applies stir bars varying in length from 1 to 4 cm coated with a relatively thick layer (0.3-1 mm) of polydimethylsiloxane (PDMS) resulting in PDMS volumes varying from 55 µL to 220 µL. After a certain stirring time, the stir bar is removed from the aqueous sample and thermally desorbed on-line with a gas chromatograph. Due to the much larger volume of the PDMS-phase extraction efficiency is far better than for SPME. Stir bar sorptive extraction (SBSE) is a solid phase extraction (SPE) method in which the sorbent is a coating of a solid magnetical stir bar. SBSE applies stir bars varying in length from 1 to 4 cm coated with a relatively thick layer (0.3-1 mm) of polydimethylsiloxane (PDMS) resulting in PDMS volumes varying from 55 µL to 220 µL. After a certain stirring time, the stir bar is removed from the aqueous sample and thermally desorbed on-line with a gas chromatograph. Due to the much larger volume of the PDMS-phase extraction efficiency is far better than for SPME.
|
| |
|
A method employing an electron microscope and a finely focused beam of electrons that is moved across a sample allowing the surficial textures to be examined at high resolution and the image displayed. By collecting the emitted electrons from a single spot (size 1-10 microns)
chemical analysis of portions of the sample, i.e. a specific mineral species, can be made using energy dispersive x-ray analysis (SEM/EDXA).
|
| |
|
|
Technique belonging to the group of scanning probe microscopy (SPM) used to directly observe individual atoms on surfaces. In STM a solid specimen in air, liquid or vacuum is scanned by a sharp tip located within a few Å from the surface. A quantum -mechanical tunneling current flows between atoms on the surface and those on the tip. The magnitude of the current depends upon the separation between the surface and tip atoms, so that it is possible to obtain surface topography with atomic resolution.
|
| |
|
|
A naturally occurring organoselenium compound found in many plants,
including garlic, onions, and broccoli, with potential antioxidant and
chemopreventive activities. Se-Methyl-seleno-L-cysteine (MSC) is an
amino acid analogue of cysteine in which a methylselenium moiety
replaces the sulphur atom of cysteine. This agent acts as an
antioxidant when incorporated into glutathione peroxidase and has been
shown to exhibit potent chemopreventive activity in animal models.
|
| |
|
Se-methylselenoneine is a Se-metabolite found in human urine most likely as a result of methylation of dietary selenoneine. Higher concentrations of this species in human urine are probably associated with the consumption of tuna which is a rich source of selenoneine.

|
| |
|
|
Standard whose value is assigned by comparison with a primary standard of the same quantity.
|
| |
|
|
Particulate material consisting of eroded soil and rock material, and plant debris,
transported and deposited by water.
|
| |
|
|
The water that occupies the spaces between sediment particles.
|
| |
|
|
SdFFF is a set of high resolution liquid chromatography-like elution methods used for sizing and separating colloidal matter into size fractions. SdFFF separations are performed within a flat open channel, usually having a rectangular cross-section and triangular end pieces where the sample and carrier fluid enters and leaves. The mechanism for particle separation involves only physical interactions.
The sample is introduced into the channel through a septum or injection
valve, and then the flow is turned off. A centrifugal field is then
applied at right angles to the flat face of the ribbon-like channel.
This flat channel sits within a centrifuge basket and the centrifugal
field drives the particles towards the accumulation wall. There they
form equilibrium clouds whose average thickness or elevation above the
accumulation wall depends on how strongly the particles interact
with the field and also their diffusivity.
|
| |
|
Selected ion monitoring (SIM) is the practice of monitoring and recording ion currents at one or more selected ion m/z values with time, rather than recording full mass spectra, as sample is introduced into the ion source. Because the detector is integrating signal for a longer time at the relevant ion, limits of detection can be lowered, albeit at a cost of susceptibility of the experiment to unexpected interferences. Use of the terms multiple ion detection, multiple ion (peak) monitoring, and mass fragmentography have also been used but are discouraged. The terms single ion monitoring or multiple ion monitoring are sometimes used.
  
|
| |
|
|
Selected reaction monitoring (SRM) is used to describe a mode of data
acquisition in tandem mass spectrometry where precursor and product ions
are selected in the first and second stages of mass spectrometry,
respectively
|
| |
|
Qualitative – the extent to which other substances interfere with the determination of a substance according to a given procedure.
Quantitative – a term used in conjunction with another substantive (e.g. constant, coefficient, index, factor, number, etc.) for the quantitative characterization of interferences.
|
| |
|
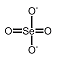 Selenates are analogous to sulfates and have similar chemistry. Unlike sulfate, selenate is a somewhat good oxidizer; it can be reduced to selenite or selenium. In strongly acid conditions, hydrogen selenate ion, HSeO 4-, is formed; the selenic acid, H 2SeO 4, is very strong |
| |
|
|
Selenic acid, H2SeO4, is an oxygen acid of selenium. Selenic acid is analogous to sulfuric acid and has similar properties; however, it is a stronger oxidizer.
|
| |
|
|
The selenide ion is Se2-. A selenide is a chemical compound in which selenium serves as a anion with oxidation number of -2, much as sulfur does in a sulfide.
|
| |
|
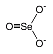
|
| |
|
Selenium is a chemical element with atomic number 34, with the chemical symbol Se. Selenium occurs only rarely in the free state in nature. It is a nonmetal that is chemically related to sulfur and tellurium. It is toxic in large amounts, but trace amounts of it, forming the active center of certain enzymes such as glutathione peroxidase(GSH-Px), are necessary for the function of all cells in (probably) all animals. Selenium requirements in plants differ by species, with some plants apparently requiring none.
Isolated selenium occurs in several different forms, but the most stable of these is a dense purplish-gray semimetal (semiconductor) form that is structurally a trigonal polymer chain. It conducts electricity better in the light than in the dark, and is used in photocells (see allotropic section below). Selenium also exists in many nonconductive forms: a black glass-like substance, as well as several red crystalline forms built of eight-membered ring molecules, like its lighter cousin sulfur.
Selenium is found in economic quantities partially replacing sulfur in sulfide ores such as pyrite. Minerals that are selenide or selenate compounds are also known, but all are rare.
|
| |
|
|
Amino acids such as selenocysteine and selenomethionine that contain selenium instead of sulfur in their structure.
|
| |
|
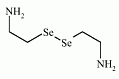 The organoselenium compound selenocystamine has been used in some trials as a Se-supplement for its anticarcinogenic activity. The organoselenium compound selenocystamine has been used in some trials as a Se-supplement for its anticarcinogenic activity.
|
| |
|
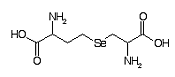 Selenocystathionine Formula: C7H14N2O4Se
Mass 270.0119
|
| |
|
|
Selenocysteine is an amino acid that is present in several selenoproteins (seleno-enzymes) in plants and animals (for example glutathione peroxidases, tetraiodothyronine 5' deiodinases, thioredoxin reductases, formate dehydrogenases, glycine reductases and some hydrogenases).
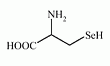 Selenocysteine has a structure similar to cysteine, but with an atom of selenium taking the place of the usual sulfur. Selenium is incorporated into amino acid sequences of selenoproteins by the specific codon to SeCys residue. |
| |
|
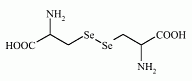 The selenoamino acid selocystine has been found in seagull eggs. The selenoamino acid selocystine has been found in seagull eggs.
|
| |
|
Selenols are organic compounds that contain the functional group with the connectivity C–Se–H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. The best known member of the group is the amino acid selenocysteine.
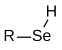
|
| |
|
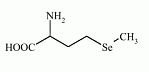 Selenomethionine is an selenoamino acid (amino acid containing selenium). The L-isomer of selenomethionine, known as Se-met, is a common natural food source of selenium. It can not be synthesized by higher animals, but can be obtained from plant material.
Chemical formula: C5H11NO2Se
IUPAC name: 2-amino-4-methylselanyl-butanoic acid
|
| |
|
A histidine derivative that is Nα,Nα,Nα-trimethyl-L-histidine substituted by a selenoxo group at position 2 on the imidazole ring. This selenium-containing antioxidant was found in tuna blood and is a major selenium compound in fish muscle. Ths compound has strong antioxidant capacity and binds to heme proteins, such as hemoglobin and myoglobin, to protect them from iron auto-oxidation, and it reacts with radicals and methylmercury (MeHg).

Formula: C9H15N3O2Se
Mol. Weight: 276.19000
|
| |
|
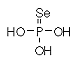 Formula H3O3PSe Formula H3O3PSe
Mass 161.8985
|
| |
|
|
A selenoprotein is any protein that includes a selenocysteine residue. Selenoproteins exist in all major forms of life, eukaryote, eubacteria and archaea. Among eukaryotes, selenoproteins appear to be common in animals, but rare or absent in other phyla (one has been identified in the green alga Chlamydomonas, but none in other plants or in fungi). Among eubacteria and archaea, selenoproteins are only present in some lineages, while they are completely absent in many other phylogenetic groups.
|
| |
|
|
A glycoprotein synthesized mostly by the liver that serves as the main plasma selenoprotein. Its role is thought to involve extracellular antioxidant activity and Se transport.
|
| |
|
The part of the proteome incorporating selenoamino acids, selenomethionine and selenocysteine.
The use of the term is often limited to proteins with genetically encoded selenocysteine only.
|
| |
|
Poisoning due to excessive intake of Se. Symptoms include hair and nail loss, skin lesions, damage to the nervous, immune and reproductive systems, convulsions, paralysis, gastrointestinal and circulatory
disturbances.
|
| |
|
|
Compounds where a selenium atom replaces the endocyclic oxygen atom of a carbohydrate.
|
| |
|
|
Scanning Electron Microscopy (SEM) – A scanning electron microscope is a microscope that uses electrons as an "illuminating" medium to resolve very fine features (i.e., thousands of times magnification are typical) in a particular specimen. The concept is similar to a light microscope, but since it uses electrons that have a much smaller wavelength than light, very small features can be resolved.
|
| |
|
|
An electron beam microprobe for X-ray-fluorescence analysis. Commonly associated with electronic microscopy (scanning electron microscopy), energy-dispersive X-ray analysis permits spatially resolved measurments of the elementary composition of materials.
|
| |
|
|
The change in the response of a measuring instrument divided by the corresponding change in stimulus.
|
| |
|
|
In sequential injection manifolds, a stack of well-defined unsegmented zones is assembled in a holding coil using a pump or liquid driver and a selection device. On transporting this stack of zones to the detector, the zones penetrate one another and mixing between their components gives rise to one or more detectable species. These products are measured as the zone stack reaches a suitable (flow-through) detector. The multiposition selection valve allows sequential injection analytical systems to be extremely versatile with many changes to the methodology being achievable through software control of the system parameters rather than actual physical changes to the hardware. Each port of the valve can be dedicated to a specific purpose, and the combinations of sample, standards, reagents, and detectors around the valve are easily modified to suit a particular analysis.
Since the system works sequential, the drawback of such versatility is a reduced sample throughput.
|
| |
|
|
In sequential leaching (extraction) procedures, chemical extractants of various types are applied to the solid sample, e.g. soil, plants, airborne particles, sludge and wastes, each successive treatment being more drastic in chemical action than the previous one.
|
| |
|
|
A batch leaching test where a solid sample is leached in successive volumes of different types of leaching solutions (compare with serial batch leaching, leaching test, column leaching, and batch leaching).
|
| |
|
|
A batch leaching test where a solid sample is leached in successive volumes of fresh aliquots of the same leaching solution (compare with sequential batch leaching, leaching test, column leaching, and batch leaching).
|
| |
|
|
The progressive dilution, by the same factor, of standard or sample in a row of tubes so that the first tube contains the highest concentration of test substance
|
| |
|
Surface Enhanced Raman Spectroscopy, or Surface Enhanced Raman Scattering, often abbreviated SERS, is a surface sensitive technique that results in the enhancement of Raman scattering by molecules adsorbed on rough metal surfaces. The enhancement factor can be as much as 1014-1015, which allows the technique to be sensitive enough to detect single molecules.
  
  
|
| |
|
|
1. Watery proteinaceous portion of the blood that remains after clotting.
2. Clear watery fluid especially that moistening the surface of serous membranes or that exuded through inflammation of any of these membranes.
|
| |
|
A liquid that is added to the sample flow towards the sample introduction system, often via a concentric capillary, to help produce a stable spray especially in techniques creating very low sample flow rates such as CE.
|
| |
|
|
A sheath flow interface is a method for coupling capillary
electrophoresis to electrospray ionization that uses a coaxial flow of
makeup liquid that is introduced through a tube that is concentric with
the separation capillary.
|
| |
|
|
Noise caused by the randomness in arrival time of ions/photons at the detector. It is proportional to the number of ions/photons arriving at the detector in a given time interval.
|
| |
|
|
Shotgun proteomics is a method of identifying proteins using a
combination of high performance liquid chromatography and mass
spectrometry in which the proteins in the mixture are digested and the
resulting peptides are separated by liquid chromatography and identified
by tandem mass spectrometry.
|
| |
|
The sensitive high-resolution ion microprobe (also sensitive high mass-resolution ion microprobe or SHRIMP) is a large-diameter, double-focusing secondary ion mass spectrometer (SIMS) sector instrument produced by Australian Scientific Instruments in Canberra, Australia. Like other SIMS instruments, the SHRIMP microprobe bombards a sample under vacuum with a beam of primary ions that sputters secondary ions that are focused, filtered, and measured according to their energy and mass.
 EVISA Instrument Database: SHRIMP EVISA Instrument Database: SHRIMP
|
| |
|
|
Siderophores (Greek: "iron carrier") are small, high-affinity iron chelating compounds secreted by organisms such as bacteria, fungi or plants. Siderophores are amongst the strongest soluble Fe3+ binding agents known.
|
| |
|
|
Magnitude
of the observed datastream related to the sum of sought-for and
interfering substances plus background, and fluctuations unrelated to
the sought-for measurements.
|
| |
|
Organosilicon compounds unlike their carbon counterparts do not have a rich double bond chemistry due to the large difference in electronegativity. Existing compounds with organosilene Si=C bonds are laboratory curiosities such as the silicon benzene analogue silabenzene. Disilenes have Si=Si double bonds and disilynes are silicon analogues of an alkyne.
|
| |
|
|
Polymerized siloxanes with organic side chains (R ≠ H) are commonly known as silicones or as polysiloxanes. Representative examples are [SiO(CH3)2]n (polydimethylsiloxane) and [SiO(C6H5)2]n (polydiphenylsiloxane). These compounds can be viewed as a hybrid of both organic and inorganic compounds. The organic side chains confer hydrophobic properties while the -Si-O-Si-O- backbone is purely inorganic.
|
| |
|
|
Siloles, members of a larger class of compounds called metalloles, are the silicon pendants of pyrroles and are of current academic interest due to their electroluminescence and other electronic properties. Siloles are efficient in electron transport. They owe their low lying LUMO to a favorable interaction between the antibonding sigma silicon orbital with a antibonding pi orbital of the butadiene fragment.
|
| |
|
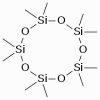 A siloxane is any chemical compound composed of units of the form R2SiO, where R is a hydrogen atom or a hydrocarbon group. A siloxane has a branched or unbranched backbone of alternating silicon and oxygen atoms -Si-O-Si-O-, with side chains R attached to the silicon atoms. A siloxane is any chemical compound composed of units of the form R2SiO, where R is a hydrogen atom or a hydrocarbon group. A siloxane has a branched or unbranched backbone of alternating silicon and oxygen atoms -Si-O-Si-O-, with side chains R attached to the silicon atoms.
The word siloxane is derived from the words silicon, oxygen, and alkane.
Fig.: Decamethylcyclopentasiloxane
|
| |
|
 Silver (I) sulfadiazine is a metallodrug used to prevent and treat bacterial or fungus infections of the skin. Silver (I) sulfadiazine is a metallodrug used to prevent and treat bacterial or fungus infections of the skin.
|
| |
|
Secondary ion mass spectrometry (SIMS) is a mass-spectrometric technique that is used for microscopic
chemical analysis. A beam of primary ions with an energy of 5- 20
kiloelectronvolts (keV) bombards a small spot on the surface of the
sample under ultra- high vacuum conditions. Positive and negative
secondary ions sputtered from the surface are analyzed in a mass
spectrometer in regards to their mass-to-charge ratio.
 EVISA Instrument Database: SIMS systems EVISA Instrument Database: SIMS systems
|
| |
|
|
The single filament method is a surface ionization method used for TIMS wherein a single filament is used to vaporize and ionize the sample.
|
| |
|
In a single-focusing mass spectrometer, a single magnetic sector is used to generate the magnetic field that differentiates ions according to their m/z values (strictly, according to their momenta). The addition of an electric sector in a specified configuration provides a double-focusing mass spectrometer that can achieve higher mass resolution than a single-focusing mass spectrometer.
  
|
| |
|
Size fractionation is the process of classification of an analyte or a
group of analytes from a certain sample acoording to molecular or
particular size.
|
| |
|
An HPLC method, different from reversed-phase chromatography, used mainly to separate high-molecular-weight samples and to determine their molecular-weight distribution. SEC, also called Gel Filtration Chromatography (GFC) or Gel Permeation Chromatography (GPC) is based on the molecular sieve effect and enables species to be separated according to their size and to a lesser extend, shape. The average time a substance spends in the pores of the packing can usually be correlated with its molecular weight.
|
| |
|
|
Analytical separation technique, often used to characterize proteins or mixtures, that uses a charged gel environment through which molecules of varying sizes and electric charges migrate from one pole to the other. Unlike gel-filtration chromatography, larger molecules move more slowly than smaller molecules because their migration rate is not dependent on diffusion into and out of particles. The SDS detergent denatures and binds to proteins, aiding in their separation.
|
| |
|
 Sodium stibogluconate belongs to the class of medicines known as the pentavalent antimonials used to treat leishmaniasis. Sodium stibogluconate is sold in the UK as Pentostam (manufactured by GlaxoSmithKline) and is only available for administration by injection. Widespread resistance has limited the utility of sodium stibogluconate, and in many parts of the world, amphotericin or miltefosine are used instead. Sodium stibogluconate belongs to the class of medicines known as the pentavalent antimonials used to treat leishmaniasis. Sodium stibogluconate is sold in the UK as Pentostam (manufactured by GlaxoSmithKline) and is only available for administration by injection. Widespread resistance has limited the utility of sodium stibogluconate, and in many parts of the world, amphotericin or miltefosine are used instead.
|
| |
|
Mass spectrometric methods leading to the formation of ions with low internal energies meant to avoid fragmentation of the molecular structure.
|
| |
|
Ionization in which a minimum of excess energy is imparted to the newly formed ion, producing little resultant fragmentation. Distinguished from high-energy ionization, sometimes called ‘hard’
ionization, typified by the products of EI.
|
| |
|
 First
generation water-soluble gold(1) thiolate metallodrug used for
treatment of rheumatoid arthritis. Serious side effects led to the
development of second generation gold drugs such as auranofin. First
generation water-soluble gold(1) thiolate metallodrug used for
treatment of rheumatoid arthritis. Serious side effects led to the
development of second generation gold drugs such as auranofin.
IUPAC name : auriothioglucose
|
| |
|
(SPE) - A sample-preparation technique that uses a solid-phase packing contained in a small plastic cartridge. The solid stationary phases are the same as HPLC packings; however, the principle is the same as in frontal chromatography. The process as most often practiced requires four steps: conditioning the sorbent, adding the sample, washing away the impurities, and eluting the sample in as small a volume as possible with a strong solvent.
|
| |
|
SPME is a solvent-free solid-phase preconcentration technique based on the sorption of analyte species present in a liquid phase or, more often in a headspace gaseous phase, on a microfiber coated with a chromatographic sorbent.
When the equilibrium is reached, the fiber is transferred to a GC injector by means of a microsyringe, and the analytes are thermally desorbed inside the heated injector.
|
| |
|
The analytical concentration of a solute in a saturated solution. The analytical concentration includes those of all the species formed by the dissolved substance in the solution. Numerical data for solubility always have to be defined in relation to the values of temperature, pressure and concentrations of other dissolved substances.
Source: IUPAC
|
| |
|
|
- analytical procedure for bringing biological materials into solution. It can be achieved by alkaline, acidic or enzymatic hydrolysis. Following the solubilization, an aqueous solution of species is obtained but the matrix is not eliminated.
|
| |
|
The minor component of a solution which is regarded as having been dissolved by the solvent.
Source: IUPAC
|
| |
|
Homogeneous liquid phase comprising at least two different substances.
Source: IUPAC
|
| |
|
A liquid (usually the major component of a solution) which is used to dissolve a solute or
solutes.
Source: IUPAC
|
| |
|
|
The removal of a soluble material from a solid mixture by means of a solvent or the removal of one or more components from a liquid mixture by use of a solvent with which the liquid is immiscible or nearly so.
|
| |
|
|
Sonic spray ionization is the process of gas phase ion formation during
atmospheric pressure droplet formation in a supersonic pneumatic spray.
Ions are formed due to a statistically unbalanced charge distribution in
the nebulized droplets
|
| |
|
A solid material that removes chemical species from liquids or gases through adsorption and/or
absorption (compare with absorbent and adsorbent). Common sorbents are polymers, silica gel, alumina, titania, zirconia, and chemically modified materials.Sorbents are used as packing materials in liquid chromatography or solid phase extraction cartridges.
|
| |
|
It is sometimes difficult or impossible to discriminate experimentally between adsorption and absorption : in such cases it is convenient to use the noncommittal term sorption. In ion exchange, sorption is the uptake of electrolytes or nonelectrolytes by ion exchangers through mechanisms other than pure ion exchange.
With respect to surface phenomena, this term is also used when the retention mechanism at a
surface is unknown. Adsorption, surface precipitation, and polymerization are all examples of sorption.
|
| |
|
Optical spectrometry:
In optical spectrometry, the source of the spectrometer is the device from which the radiation is emitted. This may be a flame or plasma source.
Mass spectrometry:
The source is the device within the mass spectrometer in which ionization of sample molecules occurs. The source may be under vacuum, or it can operate at atmospheric pressure. A chromatographic method may interface with the source, or samples may be introduced via a probe or an automated sample introduction system. Ions are accelerated out of the source into the mass analyzer of the instrument.
|
| |
|
|
The space charge effect is the mutual repulsion of
particles of like charge that limits the current in a charged-particle
beam and causes beams or packets of charged particles to expand radially
over time.
|
| |
|
Transient
plasma triggered by imposing so high a voltage across a small gap
between conducting electrodes that the intervening gas ionizes.Spark devices are used as sources both for atomiuc emission spectrometry (Spark-OES) as well as mass spectrometry (SS-MS),
|
| |
|
|
Spark source mass spectrometry is a technique using a mass spectrometer with a spark ionization source.
|
| |
|
|
Distribution of an element amongst defined chemical species in a system.
|
| |
|
|
analytical chemistry: analytical activities of identifying and /or measuring the quantities of one or more individual chemical species in a sample.
|
| |
|
|
Species-specific isotope dilution (sometimes also called speciated isotope dilution) is an isotope dilution technique using a spike compound identical to the compound under investigation but labeled with a specific isotope of the target element. Species-specific ID analysis is only possible for element species well defined in their structure and composition. The equilibration of the spike and the analyte in the sample, attainable in classical IDMS by multiple sequential dissolution and evaporation cycles, cannot be guaranteed to be achieved for species-specific ID analysis of solid samples. If applicable, the method can correct for loss of analyte during sample preparation following the spike addition, incomplete derivatisation yield and for intensity suppression/enhancement during detection.
|
| |
|
|
the class of interference related to spectral properties of components other than atomized analyte. Three sources of spectral interference can be identified including spectral line interference, scattering, and broad band spectral interference.
|
| |
|
|
Spectral skewing is the deviation of the peak intensities in a mass
spectrum from their expected values that occurs when the relative
concentration of the various analytes change during acquisition of the
mass spectrum.
|
| |
|
|
Instrument to display light of various wavelengths at separated physical positions simultaneously.
|
| |
|
|
Any instrument used to study light as a function of wavelength.
|
| |
|
An instrument to quantitatively measure the intensity of light as a function of wavelength, typically for making absorbance measurements.
|
| |
|
|
A spiked sample is a sample prepared by adding a known quantity of analyte to a matrix which is close to or identical to that of the sample of interest. Spiked samples may be used in method validation experiments to help identify matrix effects and determine the recovery of an analyte or the selectivity of the method.
|
| |
|
 Spiroplatin is a second generation metallodrug and analgue for cisplatin, developed for cancer therapy. Spiroplatin induces DNA cross-links, thereby
inhibiting DNA replication and RNA and protein synthesis. Similar to
other platinum compounds, this agent has been shown to be mutagenic and
carcinogenic.
IUPAC name: aqua-1,1-bis(aminomethyl)-cyclohexanesulfatoplatinum(II)
|
| |
|
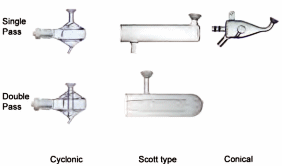 The spray chamber is part of the sample introduction system for liquid samples of a spectroscopic source such as a flame or plasma. The function of the spray chamber is to filter the aerosol produced by the nebulizer (primary/secondary aerosol) so that only the smallest reach the source (tertiary aerosol). The most commonly used spray chambers in plasma spectrochemistry are the cyclonic, barrel (or Scott-type), and conical (see figure). The spray chamber is part of the sample introduction system for liquid samples of a spectroscopic source such as a flame or plasma. The function of the spray chamber is to filter the aerosol produced by the nebulizer (primary/secondary aerosol) so that only the smallest reach the source (tertiary aerosol). The most commonly used spray chambers in plasma spectrochemistry are the cyclonic, barrel (or Scott-type), and conical (see figure).
The cyclonic and Scott-type spray chambers are available as both single and double pass (or baffled) versions. The double-pass mode acts as a secondary filter to further reduce the mean droplet site distribution (reducing the aerosol transport efficiency and reducing the noise level). The conical spray chamber uses an impact bead to break-up larger droplets. In all three designs gravity is used to remove the larger drops from the transport gas stream and divert them to the drain. Total transport efficiency is typically below 5 %. In the cyclonic spray chamber this action is assisted by centrifugal forces.
|
| |
|
|
The ejection of atoms from a surface by the impact of high-energy ions and atoms.
|
| |
|
|
Nuclei that do not decay to other isotopes on geologic timescales, but may themselves be produced by the decay of radioactive isotopes.
|
| |
|
A procedure of labeling tissues, organisms, or molecules (such as DNA or proteins) with colored or fluorescent dyes to allow visualization by microscopic or marcroscopic techniques.
|
| |
|
|
The standard-addition method is a calibration method
applied to reduce the effect of matrix interferences. It involves
adding one or more increments of a standard solution to a sample
aliquots of the same size. Each spiked sample is then diluted to a
fixed volume before analyzing. When the amount of sample is
limited, standard additions can also be carried out by successive
introductions of increments of the standard to a single measured
aliquot of the unknown. Measurements are made on the original and after
each addition. Placing a regression line through the absorptive data
points obtained from the series of additions and interpolating it till
crossing the abscissa, yields the initial analyte concentration of the
sample.
|
| |
|
A measure of dispersion or variation, usually taken as the square root of the variance.
|
| |
|
A detailed (step-by-step), instruction to achieve uniformity in the performance of a specific process or or pioece of equipment, which are approved by the quality control unit and used for GMP/GLP operations.
|
| |
|
|
Static nanoelectrospray is a variant of nanoelectrospray in which the
flow of liquid is driven by the capillary action induced by droplets
leaving the electrospray needle with no additional pumping.
|
| |
|
|
SIMS method featuring primary ion current densities (corresponding to SIMS gun flux) of 2 ~ 3 nA/cm2 or less and primarily used in analysis of sample surface components. The method is therefore distin-guished from dynamic SIMS, which is used for analysis of components in the depth direction. When the sample is an organic molecule in solid form, the term is changed to organic SIMS or molecular SIMS.
|
| |
|
The non-mobile phase in the chromatographic bed, on which the separation depends. For example, in gas solid chromatography and liquid—solid chromatography the active solid is the stationary phase, and in gas—liquid and liquid liquid chromatography the liquid, but not the solid support, is
the stationary phase.
Source: IUPAC
|
| |
|
|
Those aspects of quality
control in which statistics are applied, in contrast to the broader
scope of quality control which includes many other procedures, such as
preventive maintenance, instrument function checks, and performance
validation tests. Statistical QC procedures are often used to monitor
routine performance of a method and to alert the laboratory when the
performance of a method changes.
|
| |
|
|
1. (in chemistry): State of a system in which properties do not change with time.
2. (in toxicology): State of a system in which the conditions do not change in time.
|
| |
|
Steam distillation is a special type of distillation (a separation
process) for temperature sensitive materials that tend to decompose at
sustained high temperatures. Separation by normal distillation would
then not be an option, so water or steam are introduced into the
distillation apparatus. By adding water or steam the boiling point of
the compounds is depressed, allowing them to evaporate at lower
temperatures, preferably below the temperatures at which the
deterioration of the material becomes appreciable. If the substances to
be distilled are very sensitive to heat, steam distillation can also be
combined with vacuum distillation. After destillation the vapors are
condensed as usual, usually yielding a two-phase system of water and
the organic compounds, allowing for simple separation.
Steam distillation has been evaluated as a technique for the separation
of methylmercury from different environmental sample materials.
|
| |
|
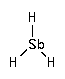 Stibine (SbH 3) is a precursor for chemical vapor deposition (CVD) of antimony atom in the production of semiconductors. In the environment, traces of stibines have been found in contaminated soils.
CAS Number : 7803-52-3
Other names: Antimony trihydride
|
| |
|
|
This is generally a standard or reagent solution of known accepted stability, which has been prepared in relatively large amounts, of which portions are used as required. Frequently, such portions are used following further dilution.
|
| |
|
|
properly refers to the set of chemical and
physical principles applied to determine the relationships between
reactants and products in a chemical process. In stoichiometric
calculations, it is the mass relationship existing between the chemical
reactants and products, which are of the primary interest. As an
adjective, "stoichiometric" is often taken to refer to the quantities
of reactants and products as determined by the reaction equation for a
specific chemical reaction, which produces no by-products.
|
| |
|
Any radiation reaching the detector that is not remitted or transmitted from, or through, the sample
at the selected measurement wavelength. In the specific case of Raman spectroscopy, stray light generally consists of the elastic stray light as Rayleigh scattering of the main laser frequency energy.
|
| |
|
Analytical efforts aiming on structural identification of molecules.
The techniques that have been developed for the identification of
chemical structures include mass spectrometry (MS), nuclear magnetic
resonance spectroscopy (NMR), infrared/ultraviolet spectrometry
(IR/UV), X-ray diffraction (XRD) and X-ray absorption fine structure
(XAFS) spectroscopy.
|
| |
|
Passing directly from a solid to a vapor state without first melting into a liquid.
|
| |
|
|
Microbes found commonly in sediments that transform inorganic mercury into organic methylmercury as a by-product of their metabolism.
|
| |
|
A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid. In addition, close to the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be "fine-tuned". Supercritical fluids are suitable as a substitute for organic solvents in a range of industrial and laboratory processes.
|
| |
|
|
An HPLC technique which employs the use of supercritical fluids as the mobile phase to enhance the separation of samples that are typically problematic for standard LC analysis.
|
| |
|
Material floating on the surface of a liquid mixture (often the liquid component that has the lowest density); the overlying fluid layer that remains after precipitation of a solid componentr through centrifugation.
|
| |
|
|
The interaction of a surface functional group with an ion or molecule present in the soil solution can create a stable molecular entity called a surface complex. The overall reaction is referred to as surface complexation.
|
| |
|
|
Water above the surface of the land, including lakes, rivers, streams, ponds, floodwater, and runoff [compare groundwater].
|
| |
|
Surface-induced dissociation (SID) is the fragmentation of an ion induced by an energetic collision of that ion with a solid surface, which can be placed between two mass analyzers. The surface then takes the place of the neutral gas molecule that is the collision target in collision-induced dissociation.
  
|
| |
|
Any substance that changes the characteristics of a surface, such as lowering the surface tension of water.
|
| |
|
|
Mineral and/or organic particles in suspension in natural or polluted waters.
|
| |
|
Particles floating in (not necessarily on) a liquid medium, or the mix of particles and liquid itself.
|
| |
|
|
Pharmacological or toxicological interaction in which the combined biological effect of two or more substances is greater than expected on the basis of the simple summation of the toxicity of each of the individual substances
|
| |
|