The U.S. Food and Drug Administration announced on Wednesday that Alpharma, a subsidiary of Pfizer Inc., will voluntarily suspend U.S. sales of the animal drug 3-Nitro (Roxarsone), a product used by poultry producers since the 1940s, in response to FDA data.
Background:
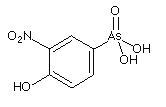
Figure: Structure of Roxarsone
|
In 1944, 3-Nitro became the first arsenic-containing new animal drug product approved by the FDA. It is used primarily in broiler chickens. Combined with other animal drugs, 3-Nitro has been used by some in the poultry industry to help control coccidiosis, a parasitic disease that affects the intestinal tracts of animals. It has also been used for weight gain, feed efficiency and improved pigmentation. The product is also sold in Canada, Mexico, Malaysia, Indonesia, the
Philippines, Vietnam, Chile, Argentina, Peru, Venezuela, Brazil,
Australia, Pakistan and Jordan. According to information given by the National Chicken Council, Roxarsone is assumed to be the "treatment of choice", though it is not administerd to organic chickens representing only about 1% of the 8.7 billion broilers produced each year in the USA. The European Union banned its use in food animals already in 1999. Published scientific reports had indicated that organic arsenic, a less
toxic form of arsenic and the form present in 3-Nitro could transform
into inorganic arsenic, a known carcinogen (see the EVISA News section below).
FDA study results:Motivated by the published scientific reports about the transformation of Roxarsone in more harmful inorganic arsenic compounds (EVISA reported about that, see below), FDA initiated its own research project in September 2009 and developed analytical methodology for the ultra trace speciation analysis of arsenic in chicken tissue. This test method uses state-of-the-art instrumentation (ion
chromatography inductively coupled plasma mass spectrometry) to identify
and measure very low levels (less than 10 ppb) of inorganic arsenic in
the presence of much higher concentrations (nearly 2 ppm or 2000 ppb) of
3-Nitro® (Roxarsone). This method, specifically developed for the
analysis of liver samples, was evaluated and validated by a second
laboratory, FDA’s Office of Regulatory Affairs (ORA) Forensic Chemistry
Center. The study was completed in February 2011, when FDA reported that by using the new method, FDA scientists found that the levels of inorganic arsenic in the livers of chickens treated with 3-Nitro
® were increased relative to levels in the livers of the untreated control chickens. FDA officials stress that the levels of inorganic arsenic detected were
very low and that continuing to eat chicken as 3-Nitro is suspended from
the market does not pose a health risk.
“FDA detected increased levels of inorganic arsenic in the livers of
chickens treated with 3-Nitro, raising concerns of a very low but
completely avoidable exposure to a carcinogen,” said Michael R. Taylor,
FDA deputy commissioner for foods. “We are pleased to announce that the
company is cooperating with us to protect the public health.”
Alpharma, a subsidiary of Pfizer, Inc., decided to voluntarily suspend sale of 3-Nitro
®
and to facilitate an orderly process for suspending use of the product
in the United States. Alpharma’s plan provides for continued sales of
3-Nitro
® for 30 days from June 8, 2011. The company
stated that allowing sales for this period will provide time for animal
producers to transition to other treatment strategies and will help
ensure that animal health and welfare needs are met. FDA officials
stress that the levels of inorganic arsenic detected were very low and
that continuing to eat chicken as 3-Nitro
® is suspended from the market does not pose a health risk.
Food safety advocacy groups see the move as a step in the right
direction, but they are urging FDA to remove all arsenic containing
compounds from animal feed. "These include Pfizer's own feed additives
containing nitarsone, as well as those containing arsanilic acid and
carbarsone," says Paige Tomaselli, staff attorney with the
Center for
Food Safety.
 Related information
Related information U.S. Food and Drug Administration: 3-Nitro (Roxarsone) and Chicken
U.S. Food and Drug Administration: 3-Nitro (Roxarsone) and Chicken U.S. Food and Drug Administration: Study on 3-Nitro (Roxarsone) and Chicken
U.S. Food and Drug Administration: Study on 3-Nitro (Roxarsone) and Chicken U.S. Food and Drug Administration: Questions and Answers Regarding 3-Nitro (Roxarsone)
U.S. Food and Drug Administration: Questions and Answers Regarding 3-Nitro (Roxarsone) David Wallinga, Playing Chicken: Avoiding Arsenic in Your Meat, Report, IATP, Minneapolis, Minnesota, USA, 2006
David Wallinga, Playing Chicken: Avoiding Arsenic in Your Meat, Report, IATP, Minneapolis, Minnesota, USA, 2006 Related studies
Related studies
Peilong Wang, Genlong Zhao, Jing Tian and Xiaoou Su,
High-Performance Liquid
Chromatography−Inductively Coupled Plasma Mass Spectrometry Based Method
for the Determination of Organic Arsenic Feed Additives and Speciation
of Anionic Arsenics in Animal Feed,
J. Agric. Food Chem.,
58/9 (2010) 5263–5270.
doi: 10.1021/jf1001205
D. Xie,
J. Mattusch,
R. Wennrich,
Separation
of Organoarsenicals by Means of Zwitterionic Hydrophilic Interaction
Chromatography (ZIC®-HILIC) and ParallelICP-MS/ESI-MS Detection,
Engineer. Life Sci., 8/6 (2008) 582-588.
doi:
10.1002/elsc.200800041 Jianjing Liu, Hongxia Yu, Haibin Song, Jing Qiu, Fengmei Sun,
Ping Li and Shuming Yang, Simultaneous
determination of p-arsanilic acid and roxarsone in feed by liquid
chromatography-hydride generation online coupled with atomic
fluorescence spectrometry
Jianjing Liu, Hongxia Yu, Haibin Song, Jing Qiu, Fengmei Sun,
Ping Li and Shuming Yang, Simultaneous
determination of p-arsanilic acid and roxarsone in feed by liquid
chromatography-hydride generation online coupled with atomic
fluorescence spectrometry,
J. Environ. Monit., 10 (2008) 975-978.
DOI: 10.1039/b803210f
Ellen K. Silbergeld, Keeve Nachman,
The Environmental and Public Health Risks Associated with Arsenical Use in Animal Feeds, Ann. N.Y. Acad. Sci., 1140 (2008) 346-357.
doi: 10.1196/annals.1454.049
F.T. Jones,
A Broad View of Arsenic, Poult. Sci., 86 (2007) 2-14.
DOI: 10.1093/ps/86.1.2
John F. Stolz, Eranda Perera, Brian Kilonzo, Brian Kail, Bryan Crable,
Edward Fisher, Mrunalini Ranganathan, Lars Wormer, Partha Basu,
Biotransformation of 3-Nitro-4-hydroxybenzene Arsonic Acid (Roxarsone) and Release of Inorganic Arsenic by Clostridium Species, Environ. Sci. Technol., 41/3 (2007) 818–823.
DOI: 10.1021/es061802i
 Spiros A. Pergantis
Spiros A. Pergantis, Edward N. Heithmar, Thomas A. Hinners,
Speciation
of Arsenic Animal Feed Additives by Microbore High-Performance Liquid
Chromatography with Inductively Coupled Plasma Mass Spectrometry, Analyst, 122/10 (1997) 1063-1068.
DOI: 10.1039/a702691i 
John R. Dean,
Les Ebdon, Michael E. Foulkes, Helen M. Crews, Robert C. Massey,
Determination
of the Growth Promoter, 4-Hydroxy-3-Nitrophenyl-Arsonic Acid in Chicken
Tissue by Coupled High-performance Liquid Chromatography-Inductively
Coupled Plasma Mass Spectrometry, J. Anal. At. Spectrom., 9/5 (1994) 615-618.
doi: 10.1039/JA9940900615 Related EVISA Resources
Related EVISA Resources Link database: Toxicity of inorganic arsenic
Link database: Toxicity of inorganic arsenic Link database: Use of organic arsenic compounds
Link database: Use of organic arsenic compounds
 Related EVISA News (newest first)
Related EVISA News (newest first)
 January 11, 2007: More evidence linking chicken litter and toxic arsenic
January 11, 2007: More evidence linking chicken litter and toxic arsenic April 6, 2006: Testing finds: Arsenic added to feedstuff finds its way into chicken meat
April 6, 2006: Testing finds: Arsenic added to feedstuff finds its way into chicken meat April 27, 2005:Conflict raised in chicken arsenic debate
April 27, 2005:Conflict raised in chicken arsenic debate February 8, 2005: The use of arsenic in "poultry industry"
February 8, 2005: The use of arsenic in "poultry industry"last time modified: February 25, 2025