A group of researchers from the UK and Iraq report on studies on the arsenic uptake by rice plant grown within a high arsenic-containing environment down to the cellular (DNA) level. They claim to have detected arsenic associated with, or integrated within, the DNA fractions.
Background:While arsenic is often used as a synonym for "poison", the toxicity of different arsenic species differs significantly. In general, inorganic arsenic species (InAs(III) and InAs(V)), are more toxic than organic species. Unfortunately, inorganic As species are often the dominant forms of As in terrestrial plants. Mainly as a consequence of the paddy soil growing conditions, rice is accumulating more arsenic from the soil than other cereals. Arsenic speciation is also an essential parameter when assessing As uptake mechanism by the plant. It is well known that InAs(V), which is a phosphate analogue, may enter the root symplast through phosphate transport proteins. Despite many studies of the arsenic uptake by plants, few studies have covered the cellular level compartmentalisation of arsenic species from root via stem to leaf and flower/grain together with its DNA-associated arsenic content.
The new study:
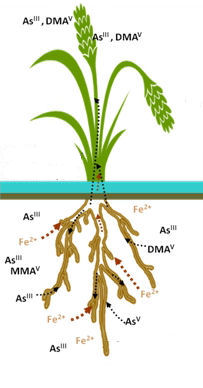
The group of researchers from the UK and Iraq report on studies on the arsenic uptake by rice plant grown within a high arsenic-containing environment down to the cellular (DNA) level. For this reason, ICP-OES, ICP-MS and HPLC-ICP-MS were used to measure the total and species concentration of As and P in cellular fractions. The highest concentration of As was found in the root of the plant and the lowest in the grain. Levels of InAs(III) and InAs(V) were identified both in soil and plant materials, while organo-arsenic species such as dimethyl arsinic acid (DMA) and monomethyl arsonic acid (MMA) were below the limit of detection. Further, a method was developed for the extraction of plant DNA to determine the different forms of As associated with, or integrated within, the DNA fractions. Measurement of As in the DNA extracts were above the LOD (0.019 µg/kg ) for the root, stem and leaf samples. The researchers observed that the concentration of both weakly and strongly associated As with DNA obtained from the root, stem and leaf decreased with decreasing total As concentrations. A near-constant ratio for the strongly associated As value (As/total As DNA) in all root, stem and leaf DNA samples (41.3 ± 0.3%) was further considered to support evidence for the incorporation of As into the DNA. The authors also admitted, that to ascertain in which form arsenic could become strongly incorporated with DNA would require further work.
An operationally defined fractionation procedure seldom if ever has the separation power to differentiate a species (e.g. As-DNA) in presence of a big excess of another species (e.g. InAs). Therefore, the presence of arsenic in the DNA fraction is not sufficient to prove that As is used to build the DNA. The authors indeed admit, that further work would be needed to demonstrate that the arsenic in the DNA is actually in the backbone. However, it is not only the poor selectivity of the detection procedure used, that is fuelling doubts on the use of arsenic instead of phosphorus in the backbone of the DNA.
It is not the first time that such results lacking convincing selectivity have been reported. In 2010, Wolfe-Simon et al. claimed to have isolated a bacterium that substitutes arsenic for phosphorus on its macromolecules and metabolites. The two atoms have very similar chemical properties, but bonds with arsenic are known to be much less stable than those with phosphate, so most researchers think that biological molecules containing arsenic rather than phosphorus would be too unstable to support life. Anyhow, the results were disputed and
taken apart quite thoroughly by Rosie Redfield, who tried to duplicate the results and failed to detect any arsenic in the DNA.
If not included into the backbone of the DNA, could arsenic bind to DNA ? This is a question that has been studied for a long time. Toxicologists gave the answer: Arsenite does not bind to DNA.
So then the questions is, what species is arsenic weakly or strongly associated with rice plant DNA ? Unfortunately, the authors fail to tell us.
Michael Sperling
 The original study:
The original study:
 Mike E. Foulkes
Mike E. Foulkes, Bashdar A. Sadee,
Steve J. Hill,
Arsenic speciation and its DNA fractionation in the rice plant Oryza sativa, J. Anal. At. Spectrom., 35/9 (2020) 1989-2001:
DOI: 10.1039/D0JA00141D
 Used techniques and instrumentation:
Used techniques and instrumentation:
 Related studies (oldest first)
Related studies (oldest first)

F. Wolfe-Simon, P.C.W. Davies, A.D. Anbar,
Did nature also choose arsenic ?, Int. J. Astrobiol., 8/2 (2009) 69-74.
DOI: 10.1017/S1473550408004394
F. Wolfe-Simon, J.S. Blum, T.R. Kulp, G.W. Gordon, S.E. Hoeft, J. Pett-Ridge, J.F. Stolz, S.M. Webb, P.K. Weber, P.C.W. Davies, A.D. Anbar and R.S. Oremland,
A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus, Science, 332 (2011) 1163–1166.
DOI: 10.1126/science.1197258.

Stefan Oehler,
Comment on ''A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus", Science, 332 (2011) 1149.
DOI: 10.1126/science.1201381

David W. Borhani,
Comment on ''A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus", Science, 332 (2011) 1149.
DOI: 10.1126/science.1201255

Steven A. Benner, Comment on
''A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus", Science, 332 (2011) 1149.
DOI: 10.1126/science.1201304

P.L. Foster,
Comment on ''A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus", Science, 332 (2011) 1149.
DOI: 10.1126/science.1201551

F. Wolfe-Simon, J.S. Blum, T.R. Kulp, G.W. Gordon, S.E. Hoeft, J. Pett-Ridge, J.F. Stolz, S.M. Webb, P.K. Weber, P.C.W. Davies, A.D. Anbar and R.S. Oremland,
Response to Comments on ''A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus", Science, 332 (2011) 1149i.
DOI: 10.1126/science.1202098

Mostafa I. Fekry, Peter A. Tipton, and Kent S. Gates,
Kinetic Consequences of Replacing the Internucleotide Phosphorus Atoms in DNA with Arsenic, ACS Chem. Biol., 6/2 (2011) 127-130.
DOI: 10.1021/cb2000023

Barry P. Rosen, A. Abdul Ajees, Timothy R. McDermott,
Life and death with arsenic; Arsenic life: An analysis of the recent report ‘‘A bacterium that can grow by using arsenic instead of phosphorus’’, Bioessays, 33 (2011) 350–357.
DOI: 10.1002/bies.201100012

Elizabeth J. Denning and Alexander D. MacKerell,
Impact of Arsenic/Phosphorus Substitution on the Intrinsic Conformational Properties of the Phosphodiester Backbone of DNA Investigated Using ab Initio Quantum Mechanical Calculations, J. Am. Chem. Soc., 133 (2011) 5770–5772.
DOI: 10.1021/ja201213b

Arnost Mladek, Jirí Sponer, Bobby G. Sumpter, Miguel Fuentes-Cabrera, Judit E. Sponer,
Theoretical modeling on the kinetics of the arsenate-ester hydrolysis: implications to the stability of As-DNA, Phys. Chem. Chem. Phys., 13 (2011) 10869–10871.
DOI: 10.1039/C1CP20423H

Jing Wang, Jiande Gu, Jerzy Leszczynski,
Could hydrolysis of arsenic substituted DNA be prevented? Protection arises from stacking interactions, Chem. Commun., 48 (2012) 3626–3628.
DOI: 10.1039/c2cc16600c
Marshall Louis Reaves, Sunita Sinha, Joshua D. Rabinowitz, Leonid Kruglyak, Rosemary J. Redfiels,
Absence of Detectable Arsenate in DNA from Arsenate-Grown GFAJ-1 Cells, Science, 337/6093 (2012) 470-473.
DOI: 10.1126/science.1219861 Related Information
Related Information Rosie Redfield Research Blog (May 28, 2011): How might a bacterium evolve to use arsenic in place of phosphorus?
Rosie Redfield Research Blog (May 28, 2011): How might a bacterium evolve to use arsenic in place of phosphorus?
 Nature News (August 9, 2011): Open research casts doubt on arsenic life
Nature News (August 9, 2011): Open research casts doubt on arsenic life
 Related EVISA News
Related EVISA News (newest first)
last time modified: September 21, 2024