|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
Dalton (Da) is a newer unit of mass taken as identical to u (the unified atomic mass unit), but not accepted as standard nomenclature by the IUPAC or IUPAP. The dalton or u is equal in mass to 1/12 the mass of a 12C atom. Mass is often expressed by biologists as kilodaltons and abbreviated kDa, and this unit sometimes appears as the label on the x-axis of a mass spectrum. d
  
  
|
| |
|
A small-molecule organic arsenical with potential antineoplastic activity. Although the exact mechanism of action is unclear, darinaparsin, a highly toxic metabolic intermediate of inorganic arsenicals (iAs) that occurs in vivo, appears to generate volatile cytotoxic arsenic compounds when glutathione (GSH) concentrations are low. The arsenic compounds generated from darinaparsin disrupt mitochondrial bioenergetics, producing reactive oxygen species (ROS) and inducing ROS-mediated tumor cell apoptosis; in addition, this agent or its byproducts may initiate cell death by interrupting the G2/M phase of the cell cycle and may exhibit antiangiogenic effects. Compared to inorganic arsenic compounds such as arsenic trioxide (As2O3), darinaparsin appears to exhibit a wide therapeutic window.
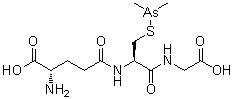
Name: Darinaparsin
IUPAC/Chemical name: (S)-2-amino-5-(((R)-1-((carboxymethyl)amino)-3-((dimethylarsino)thio)-1-oxopropan-2-yl)amino)-5-oxopentanoic acid
CAS#: 69819-86-9
Chemical Formula: C12H22AsN3O6S
Exact Mass: 411.04453
Molecular Weight: 411.31
Elemental Analysis: C, 35.04; H, 5.39; As, 18.22; N, 10.22; O, 23.34; S, 7.80
|
| |
|
|
The background signal from a detector in the absence of an instrument response to a sample.
|
| |
|
|
Direct analysis in real time by mass spectrometry; it is a proprietary term indicating the formation of ions from a solid or liquid sample at atmospheric pressure through the interaction of a gas stream containing internally excited atoms or molecules with the surface.
|
| |
|
|
Data-dependent acquisition is a mode of data collection in tandem mass
spectrometry in which a fixed number of peaks selected from a survey
scan using predetermined rules are selected and the corresponding ions
are subjected to MS/MS analysi.
|
| |
|
|
Ion generated by the cleavage of a specific parent ion. Although all fragment ions are product ions of specific parent ions, not all product ions are fragment ions. Refer to the fragment ion definition.
|
| |
|
|
Plasma source with current running in a single direction. Commonly used for atomic emission analysis of
powdered solids or metals. Cathode is primarily sampled electrode.
|
| |
|
|
A two or three electrode DC arc optimized for analysis of aerosols and sprayed solutions.
|
| |
|
The time after acceptance of a detected ion before a subsequent ion can be detected.
|
| |
|
|
Extra volume experienced by solutes as they pass through a chromatographic system. Excessive dead volume causes additional peak broadening.
|
| |
|
The minimum time between two events, that can be detected by the
detector. After a pulse, the detector is dead for a short moment before
ready for the next count.
|
| |
|
|
general term for the removal of alkyl group from a compound
|
| |
|
|
A definitive method is a method of exceptional scientific status which is sufficiently accurate to stand alone in the determination of a given property for the certification of a reference material. Such a method must have a firm theoretical foundation so that systematic error is negligible relative to the intended use. Analyte masses (amounts) or concentrations must be measured directly in terms of the base units of measurements, or indirectly related through sound theoretical equations. Definitive methods, together with certified reference materials, are primary means for transferring accuracy, i.e. establishing traceability.
|
| |
|
Delayed extraction is an experimental technique in time-of-flight mass spectrometry in which improved mass resolution is obtained by using a controlled time delay between the initial pulse of ion formation and acceleration of the ions into the flight tube of the instrument. The technique is also called time-lag focusing.
  
|
| |
|
|
The removal of a methyl (–CH3) from a chemical species (compare with methylation).
|
| |
|
|
Dental amalgam is a combination of
mercury with other metals and has been used
for over 150 years for the treatment of
tooth cavities because it is very strong
and durable.
Dental amalgams are made by mixing one part of liquid mercury with one part of
a mixture of other metals: mainly silver, but also tin, some copper and small
amounts of zinc.
|
| |
|
|
The removal of phosphate group from an organic compound by hydrolysis is called dephosphorylation. It is a physiological way to activate certain in vivo enzymes.
|
| |
|
Uranium having a percentage of uranium-235 smaller than the 0.7 percent found in natural uranium. It is obtained from spent (used) fuel elements or as byproduct tails, or residues, from uranium isotope separation.
|
| |
|
|
monitoring of signal intensity as a function of a variable that can be related to distance normal to the surface
|
| |
|
|
Analytical process of the controlled conversion of species originally present in a sample into forms with improved chromatographic yield or separation coefficient. The most popular is derivatization of ionic or highly polar species into non-polar species that can be readilly separated by GC (e.g. Grignard derivatization).
|
| |
|
|
Desferrioxamine (DFO) - Chelating agent used world-wide in the
treatment of iron overload conditions, such as hemochromatosis and
thalassemia.
|
| |
|
Design of Experiments (DOE) is an optimal
method for planning scientific experimentation.
The use of DOE ensures maximum information return for minimum investment in time and resources. DOE selects a diverse and representative set of experiments in which all factors are independent of each other despite being varied simultaneously. The result is a causal predictive model showing the importance of all factors and their interactions. These models can be summarized as informative contour plots highlighting the optimum combination of factor settings.
|
| |
|
The desolvation chamber is the enclosure, often heated, that houses the
sprayer and drying gasses in an electrospray or other spray-based ion
source or sample introduction unit.
|
| |
|
|
A device to remove solvent from the aerosol generated by the nebulizer.
|
| |
|
|
Release of a bound chemical compound from a solid surface (the opposite of adsorption)
|
| |
|
|
An ionisation technique that takes place outside of the mass spectrometer, at ambient temperature and pressure. A fine spray of charged droplets, formed by pneumatically assisted electrospray ionisation at high potential, typically 2-5 kV, is directed towards the sample on a surface. Ions produced from the sample are drawn into the mass spectrometer via a vacuum interface. Most surfaces and analytes are amenable and no sample preparation or pre-separation is required. Analytes may be analysed in situ, such as explosives on luggage, drugs in urine and metabolites in tissue.
|
| |
|
Desorption ionization (DI) is a general term used to group various methods (secondary ion mass spectrometry, fast atom bombardment, californium fission fragment desorption, and plasma desorption) in which ions are generated directly from a sample by rapid energy input into the condensed phase sample. There may be no discrete process of desorption (in the thermal sense), but instead a transfer of usually nonvolatile sample molecules into the gas phase as ions that can subsequently be mass-analyzed.
  
|
| |
|
|
Minimum amount of the characteristic property of element that can be detected with reasonable certainty under specific measuring conditions
|
| |
|
|
Deviation is the difference between the mean and an individual result.
|
| |
|
Product ion whose formation reveals structural or compositional information of its precursor. For
instance, the phenyl cation in an electron ionization mass spectrum is a diagnostic ion for
benzene and derivatives
|
| |
|
|
in chemistry, separation of suspended colloidal particles from dissolved ions or molecules of small dimensions (crystalloids) by means of their unequal rates of diffusion through the pores of semipermeable membranes
|
| |
|
|
Dibutyltin (DBT) is used in the stabilization of the plastics polymerization process, and as a catalyst in polyvinylchloride (PVC) products. Approximately 70% of the total annual world production of non-pesticidal organotin compounds are used in the thermal and UV stabilization of PVC. Approximately 27000 tons of DBT and monobutltin (MBT) is used each year as stabilizers and catalysts. These organotin compounds make their way into the environment through PVC processing plants and PVC products maintained in water and water-handling systems. Also not used as a pesticide, DBT also finds its way into environmental systems as the primary degradation product of tributyltin (TBT), an active ingredient used as an antifoulant in marine paint.
|
| |
|
|
A dietary supplement is a foodstuff, administrated to supplement the normal diet, which is a concentrated source of vitamins or minerals, or other substances with a nutritional or physiological effect, marketed in a form that allows the dosage including capsules, tablets, and other similar forms, sachets of powder, ampoules of liquid, drop-dispensing bottles, and other similar forms of liquids and powders designed to be eaten in small, measured amounts.
|
| |
|
|
Transmitting or reflecting optical element with regularly spaced scribes or grooves
on its surface, designed to use phase-dependent constructive
interference to separate light by dispersing it at wavelength-dependent
angles.
|
| |
|
|
Diffusive gradients in thin films (DGT) samplers rely on diffusion of
metals through a permeable diffusion layer of known thickness for in
situ measurement of trace metals in natural waters, with the principal
diffusion layer used to date being based on polyacrylamide hydrogels.
|
| |
|
A chemically inert substance added to a solution to increase the volume and reduce the concentration: a diluting agent.
|
| |
|
|
The mathematical factor applied to the determined value (data obtained from a calibration graph) which allows the concentration in the original sample to be determined. Frequently, for solid samples, this will involve a sample weight and a volume to which the digested/extracted sample is made up to prior to analysis. For liquid samples, this will involve an initial sample volume and a volume to which the digested/extracted sample is made up to prior to analysis.
|
| |
|
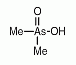 Dimethylarsinic acid (DMAV) is an organoarsenic compound that has been identified as one of the main metabolites found in urine of humans (and animals) after ingestion of inorganic arsenic compounds. Dimethylarsinic acid (DMAV) is an organoarsenic compound that has been identified as one of the main metabolites found in urine of humans (and animals) after ingestion of inorganic arsenic compounds.
|
| |
|
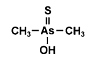 Dimethylarsinothioic acid (DMAS) also called dimethylthioarsinic acid (DMTAV) is an organoarsenic compound that has recently
been identified as human metabolite after exposure toward both the human
carcinogen inorganic arsenic and arsenosugars, which are the major
arsenical constituents of marine algae. It was earlier already identified as a metabolite in sheep's urine after ingestion of seaweed containing arsenosugars. Dimethylarsinothioic acid (DMAS) also called dimethylthioarsinic acid (DMTAV) is an organoarsenic compound that has recently
been identified as human metabolite after exposure toward both the human
carcinogen inorganic arsenic and arsenosugars, which are the major
arsenical constituents of marine algae. It was earlier already identified as a metabolite in sheep's urine after ingestion of seaweed containing arsenosugars.
|
| |
|
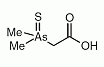 Dimethylarsinothioyl acetic acid (DMAAS) is an organoarsenic compond that has been identified as a minor metabolite in sheep's urine after ingestion of seweed containing arsenosugars. Dimethylarsinothioyl acetic acid (DMAAS) is an organoarsenic compond that has been identified as a minor metabolite in sheep's urine after ingestion of seweed containing arsenosugars.
|
| |
|
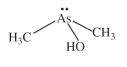 An organic trivalent arsenical with the composition of (CH3)2As(OH), abbreviated DMA(III), see Figure (compare with dimethylarsinic acid, monomethylarsonic acid, and monomethylarsonous An organic trivalent arsenical with the composition of (CH3)2As(OH), abbreviated DMA(III), see Figure (compare with dimethylarsinic acid, monomethylarsonic acid, and monomethylarsonous
acid).
|
| |
|
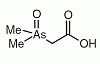 Dimethylarsinoyl acetic acid (DMAA) is an organoarsenic compound found in sheep's urine as a minor metabolite after ingestion of seaweed containing arsenosugars. Dimethylarsinoyl acetic acid (DMAA) is an organoarsenic compound found in sheep's urine as a minor metabolite after ingestion of seaweed containing arsenosugars.
|
| |
|
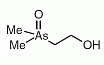 Dimethylarsinoylethanol (DMAE) was found in sheep's urine as a metabolite after seaweed consumption containing arsenosugars as the main arsenic compounds.
|
| |
|
Dimethylarsonyldimethylarsinic acid has been identified as one of the organoarsenic species present 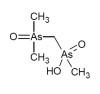 in some rice grain samples. It is present in some rice samples both as oxide and as mono- and di-thiolated compound. The thiolated forms are highly sensitive to the extraction conditions, especially the application of heat and the use of hydrogen peroxide.
|
| |
|
 Dimethyldithioarsinic acid (DMDTAV) has recently been found in landfill leachates as a microbial degradation product. Dimethyldithioarsinic acid (DMDTAV) has recently been found in landfill leachates as a microbial degradation product.
|
| |
|
|
Organomercury compound consisting of a single mercury atom and two methyl groups [(CH3)2Hg], highly volatile and not persistent in the environment. As an organic mercury compound, it may
form when mercury combines with carbon covalently (shares electrons forming a
strong bond). Dimethylmercury is poisonous to the nervous system (a neurotoxin),
it readily crosses the blood-brain barrier and causes a lack of coordination,
sensory disturbance and changes in mental state. Dimethylmercury inhibits
several stages of neurotransmission in the brain.
|
| |
|
|
(CH3)2Se The volatile organoselenium compound dimethylselenide is a metabolite in the primary pathway of the metabolism of selenite by animals and man.
|
| |
|
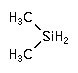 Dimethylsilane and tetramethylsilane are used as a Chemical Vapor Deposition (CVD) silicon precursor for low k dielectric layers in damascene metallization applications.
CAS Number : 1111-74-6
|
| |
|
|
(CH3)2Te - Dimethyltelluride is generated through methylation from organic and an inorganic salt of tellurium. The volatile metalloid compound has been identified in a variety of anthropogenic gases, e.g. landfill gas and sewage sludge digester gas.
|
| |
|
|
Diphenylarsinic acid (DPAA) is a degradation product of organoarsenic warfare chemicals in the environment. Due to the breakdown of some buried storage vessels or chemical warheads organoarsenic warfare chemicals can be released and DPAA can contaminate underground or well water.
|
| |
|
|
Diphenylchloroarsine (DA) is an organoarsenic compound developed near the conclusion of World War I. The chemical warfare compound is solid when pure, and has been used as an aerosol dispersed by thermal grenades or smoke generators. Its effect are: severe irritation of the eyes, nose, and throat. If the agent is inhaled for 1-2 minutes, tightness of the chest and headache are experienced. The headache develops into general nausea, which can result in vomiting in approximately three minutes. Under concentrations expected to occur under combat conditions, fatalities are not expected; however, these compounds can be fatal at higher concentrations.
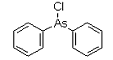 DA was used by German troops in 1917 and was considered a significant development because it penetrated the activated carbon gas mask filters deployed in World War I. Its irritant behavior was considered more important than its lethality. DA was used in combination with phosgene and diphosgene; DA caused victims to remove their masks to sneeze, cough, or vomit, rendering them vulnerable to the toxic effects of the other agents. DA alone saw some use as a riot-control agent up to the 1930s.
Names: diphenylarsinous chloride, diphenylchloroarsine, Clark-1
Molecular formula: C12H10AsCl
CAS Registry Number: 712-48-1
|
| |
|
|
Diphenylarsinous cyanide (DC) is an organoarsenic compound developed near the conclusion of World War I. The chemical warfare compound is solid when pure, and has been used as an aerosol dispersed by thermal grenades or smoke generators. Its effect are: severe irritation of the eyes, nose, and throat. If the agent is inhaled for 1-2 minutes, tightness of the chest and headache are experienced. The headache develops into general nausea, which can result in vomiting in approximately three minutes. Under concentrations expected to occur under combat conditions, fatalities are not expected; however, these compounds can be fatal at higher concentrations.
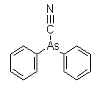 DC was used by the Germans in 1918. It was intended to combine the vomiting potential of DA with the lethality of cyanide. However, DC did not prove particularly lethal in tests. DC was a standard agent (Red No. 1) in the arsenal of the Japanese Imperial Forces between 1931 and 1945.
Names: DC, Diphenylarsinous cyanide, Clark II, diphenylcyanoarsine
Molecular formula: C13H10AsN
CAS Registry Number: 23525-22-6
|
| |
|
|
This is a variant of the direct insertion probe (or direct probe) in which the sample is coated on a surface that is inserted within the ion source of the mass spectrometer, and thus exposed to the ionization beam in the source directly. The direct exposure probe can be used to generate mass spectra of otherwise nonvolatile sample molecules.
|
| |
|
|
The direct injection nebulizer (DIN) is a microconcentric pneumatic nebulizer with no spray chamber for sample introduction to the inductively coupled plasma (ICP). The DIN is placed directly in the torch, taking the place of the injector and nebulises the liquid sample directly into the central analyte channel of the ICP. Because of the limited solvent load capacity of the plasma, the DIN must be operated at low flow rates between 30-100 µl/min.
|
| |
|
Device for introducing a single sample of a solid or liquid, usually contained in a quartz or other non-reactive sample holder, into an atomizer or ion source.
|
| |
|
The direct insertion probe is a shaft having a sample holder at one end. The probe is inserted through a vacuum lock to place the sample holder near to the ion source. The sample is vaporized by heat from the ion source or by heat from a sepa- rate heater surrounding the sample holder. The sample molecules are evaporated into the ion source where they are then ionized as gas-phase molecules.
|
| |
|
An ion detector that consists of a device that produces one or more electrons from each ion
that impacts it followed by a set of individual stages that amplify the signal by emitting more secondary electrons than electrons that impact from a prior stage.
|
| |
|
in spectroscopy: a) Change in refractive index with wavelength. b) Change in propagation direction or location of a light beam with wavelength
in flow analysis: mixing of different components of the liquid phase
|
| |
|
|
A procedure in which the mobile phase contains a compound (the Displacer) morestrongly retained than the components of the sample under examination. The sample isfed into the system as a finite slug.
|
| |
|
Mixing of two phases with the formation of one new homogeneous phase (i.e. the solution).
Source. IUPAC
|
| |
|
|
The concentration of metals contained in a sample after the sample is filtered through a 0.45 µm filter.
|
| |
|
It is known that HgII compounds in water being present in the the top surface microlayer can be reduced to produce Dissolved Gaseous Mercury (DGM) which due to its low Henry's Law constant evades from the water to the air.
|
| |
|
|
Refers to material which will pass through a 0.45μm membrane filter assembly prior to sample acidification.
|
| |
|
|
1. Apportionment of a solute between two phases. The terms parttion or extraction may also be used in this sense were appropriate.
2. Dispersal of a substance and its derivatives throughout the natural environment or throughout an organism.
3. Final location(s) of a substance within an organism after dispersal.
|
| |
|
A covalent bond formed between sulfur atoms of different cysteines in a protein; such bonds (links, bridges) help hold proteines together.
|
| |
|
|
The soluble fraction of organic matter in soils, ground and surface waters comprising low molecular weight organic compounds which have the ability to complex many elements and render them more available to plants and more prone to leaching down the soil profile.
|
| |
|
|
(of a substance): Total quantity of a substance administered to, taken up, or absorbed by an organism, organ, or tissue.
(of radiation): Energy or amount of photons absorbed by an irradiated object during a specific exposure time divided by ara or volume.
|
| |
|
The relationship between the amount of exposure [dose]
to a substance and the resulting changes in body function or health (response).
Source: ATSDR Glossary of Terms
|
| |
|
A mass spectrometer consisting of a magnetic sector and an electric sector in series which focusses ions that have differing initial momentum and kinetic energies. When the electric sector comes first, in the EB configuration, the arrangement is known as forward geometry.
Conversely, the BE configuration is known as reverse geometry. Two EB configurations are Nier-Johnson geometry and Mattauch Herzog geometry. In the former, a deflection of π/2 rad in E is followed by a deflection of π/3 rad in B to focus the ions at a focal point. In the latter, a deflection of π/4v2 rad in E is followed by a deflection of π/2 rad in B to focus the ions on a focal plane. Also called a sector mass spectrometer.
|
| |
|
|
Is a solution containing an isotopically enriched element with two isotopes with enhanced abundances in comparison to the natural abundance.
|
| |
|
A magnetic analyzer and an electric analyzer are combined in a specified geometrical configuration and sequence to accomplish both direction and velocity focusing of an ion beam from an ion source. This combination provides a higher instrumental resolving power and the ability to make more accurate mass measurements for ions.
  
|
| |
|
|
Mass spectrometer that focuses both the velocity and the speed of flying ions. Normally electric and magnetic fields are used for the focusing.
|
| |
|
|
A species that is formed when an ion is generated with a double positive charge as opposed to a normal single charge and produces an isotopic peak at half its mass. For example, the major isotope of barium at mass 138 amu also exhibits a doubly charged ion at mass 69 amu, which can potentially interfere with gallium at mass 69. Some elements such as the rare earths readily form doubly charged species, whereas others do not. Formation of doubly charged ions is also impacted by the ionization conditions (RF power, nebulizer gas flow, etc.) in the plasma discharge
|
| |
|
|
Downhole elemental fractionation is the change in the measured ratios between different elements, and occurs during laser ablation as the hole created by the laser deepens.
|
| |
|
Any substance which when absorbed into a living organism may modify one or more of its functions.
Comment: The term is generally accepted for a substance taken for a therapeutic purpose, but is also commonly used for substances of abuse. Just as any substance can be a toxicant, so any substance can be a drug. The term carries with it the implication of use for medical purposes, but also the potential for abuse to produce an effect desired by the abuser, but which is ultimately harmful.
|
| |
|
|
Use of heat to destroy the organic matrix of a sample to liberate the metal content.
|
| |
|
|
- process of
species transport from the atmosphere to the underlying surface at their
direct (without precipitation) physical-chemical interaction with elements
of the underlying surface; dry deposition is of a continuous character
independent of the occurrence or absence of atmospheric precipitation;
|
| |
|
Remaining solid material after evaporation of all water. Often used to express concentration of minerals and trace elements to eliminate variation due to differences in water content of plant material.
|
| |
|
The plasma is called to be dry when the sample fed into the plasma does not contain any solvent. This can be achieved when introducing solid samples via arc- or laser ablation, electrothermal vaporization, powder dispersion, gas chromatography or aerosol generation with desolvation systems.
|
| |
|
|
A pulsed ion analyser, such as a TOF, can only sample an ion beam for a
fraction of time, defined by the period it takes for the detector to
complete one complete analysis. The overall efficiency of detection is
referred to as the duty cycle and expressed as a percentage of the total
signal admitted to the analyser. Also used to express the limited time
(dwell time) spent during a scanned spectrum on any single m/z value
compared to that for a single ion SIM or MRM experiment (in this sense duty cycle refers to the ion collection or detection time divided by the total scan time x 100 %)
|
| |
|
The period of time that the analytical instument accumlates the signal. In sequential ICP-MS (e.g. quadrupole systems) the dwell time is characterizing the time spent for acquiring data at each of the channels which make up a peak in the mass spectrum. The length of time is measured in fractions of a millisecond, and will ultimately affect the frequency with which data is acquired at each mass. This will have a bearing on the final precision of the isotope ratio because of the influence of various sources of noise on the analytical signal.
During SIM, the length of time allocated for measuring a particular ion or ions. It can be adjusted so that specified ions can be measured for longer periods, increasing the sensitivity of detection. The term is also used in MRM to describe the time taken to analyse a particular transition. In an ion trap CID experiment, the dwell time is adjusted to increase or decrease the collision time with the added gas.
|
| |
|
|
Ratio of
the largest quantity reliably measured by a technique to the lowest
quantity so measured. A method with detection limit 1 ppb and upper
concentration of working curve linearity of 100 ppm has a dynamic range
of 100 ppm / 0.001 ppm = 106
|
| |
|
The collision reaction cell known by the trade name dynamic reaction cell (DRC) was introduced by Perkin-Elmer on their Elan DRC (followed by Elan DRC II and Elan DRC-e) instrument. The DRC is a chamber placed before the traditional quadrupole chamber of an ICP-MS device, for eliminating isobaric interferences. The chamber has a quadrupole and can be filled-up with reaction (or collision) gases (ammonia, methane, oxygen or hydrogen), with one gas type at a time or a mixture of two of them, which reacts with the introduced sample, eliminating some of the interference.
The DRC is characterized by the following parameters, that can be modified: RPq (the corresponding q parameter from the Mathieu equation), RPa (the corresponding a parameter from the Mathieu equation), which refer to the voltage applied to the quadrupole rods and the gas flow of the reaction gas.
Ammonia gas is the best solution for the majority of interferences, but it is far for being the perfect gas. Sometimes, for specific isotopes, other gas must be used for better results or even mathematical correction, if no gas offers a satisfactory advantage.
|
| |
|
|
SIMS analysis with primary ion current density (corresponding to SIMS gun flux) of several µA/cm2 or more. Primarily used in component analysis of sample surface layers in the depth direction.
|
| |
|