For the first time a compound with mercury in the +4 oxidation state has been synthesized. While theoretical work has long predicted that mercury could be stable in the +4 oxidation state, up to now mercury was generally considered to be limited to the +1 and +2 oxidation states.
In a fundamental advance theory has now been confirmed with the successful synthesis of mercury tetrafluoride (HgF
4) using matrix isolation techniques. The Hg(IV) compound was prepared under cryogenic conditions by reacting mercury with an excess of fluorine in either an argon or a neon matrix, with ultraviolet irradiation from a mercury arc lamp.
According to chemistry professor
W. Lester S. Andrews at the
University of Virginia, who did the experimental work with senior research scientist Xuefeng Wang, two factors were critical to the success of the experiments. These were controlling the light intensity, since HgF
4 is photosensitive, and using neon to improve the yield. Neon has a lower melting point than argon and thus is more permeable to fluorine at 4 K and gives higher yields of HgF
4.
Andrews and Wang confirmed the identity of the species by comparing experimental infrared spectra with vibrational frequencies predicted by coupled-cluster and density functional calculations. The theoretical work was done by Sebastian Riedel, now a postdoc at the
University of Helsinki, in Finland, and chemistry professor Martin Kaupp at the
University of Würzburg, in Germany.
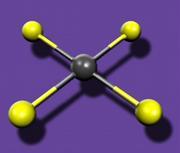
Although the researchers have not yet obtained structural data on HgF
4, prior computations by Kaupp predicted that it would be a low-spin d8 species with square-planar geometry and that the mercury d-orbitals would be strongly involved in bonding, just as in other transition metals.
Fig. 1: Mercury atom (grey) surrounded by four fluorine atoms (yellow) in a square-planar structureThe work is likely to stimulate renewed efforts to synthesize other Hg(IV) species, says Schrobilgen, who has made past attempts to synthesize high-valent mercury fluorides himself. One likely target is HgF
62–, since an anion would be better able to stabilize the high oxidation state than a neutral or cationic species. While there is no practical application yet visible, this finding has important consequences for the periodic system. Mercury, as a group 12 element with a valence electron configuration of s
2d
10 should now be considered a transition metal that can use d-orbitals for binding.
 Original study
Original studyXuefang Wang, Lester Andrews, Sebastian Riedel, Martin Kaupp,
Mercury is a Transition Metal: The First Experimental Evidence for
HgF4, Angewandte Chemie,
DOI: 10.1002/anie.200703710 Related studies
Related studiesMartin Kaupp, Hans Georg von Schnering,
Gaseous Mercury(IV) Fluoride, HgF4: An Ab Initio Study, Angewandte Chemie International Edition in English, 32/6 (1993) 861 - 863.
DOI: 10.1002/anie.199308611  Related EVISA News
Related EVISA News October 18, 2005: For the First Time, a Five-Fold Bond
October 18, 2005: For the First Time, a Five-Fold Bond April 27, 2004: New kind of mercury found in fish
April 27, 2004: New kind of mercury found in fish last time modified: March 10, 2024