|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
|
Adamsite (DM) is an organoarsenic compound developed near the conclusion of World War I. The chemical warfare compound is solid when pure, and has been used as an aerosol dispersed by thermal grenades or smoke generators. Its effect are: severe irritation of the eyes, nose, and throat. If the agent is inhaled for 1-2 minutes, tightness of the chest and headache are experienced. The headache develops into general nausea, which can result in vomiting in approximately three minutes. Under concentrations expected to occur under combat conditions, fatalities are not expected; however, these compounds can be fatal at higher concentrations.
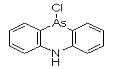 DM was discovered by German scientists in 1913 (Ger. pat. appl. 281049, July 1913 to F. Bayer and Co.), but was never used by Germany. It was independently discovered by Major Robert Adams working at the university of Illinois and also by a British team, both at the beginning of 1918. DM was produced, but not used, by the Americans at the end of the war; Franke states that "according to very incomplete reports [it] was used by the Italian Army." It was produced by many nations for use as a riot control agent until it was superseded by alpha-chloroacetophenone (CN) and similar tear agents. It was also found to be effective as a pesticide against marine borers, which kept in production for years. By 1920, gas mask filters had been improved to protect against aerosol particles, which may account for the termination of this line of development.
Names: DM, 10-Chloro-5,10-dihydrophenarsazine, Adamsite
Molecular formula: C12H9AsClN
CAS Registry Number: 578-94-9
|
| |
|
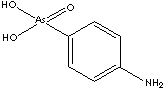 Arsanilic acid, 4-Aminophenylarsonic acid derived from orthoarsenic acid, is an arsenical antibacterial veterinary medicine used in the prevention and the treatment of swine dysentery. Arsanilic acid, 4-Aminophenylarsonic acid derived from orthoarsenic acid, is an arsenical antibacterial veterinary medicine used in the prevention and the treatment of swine dysentery.
|
| |
|
|
An arsenate (compound) is some compound that contains the arsenate ion : AsO43- . Arsenate is much like phosphate. In acid conditions we have arsenic acid, H3AsO4; in weakly acid conditions we have the dihydrogen arsenate ion, H2AsO4-; in weakly basic conditions we have hydrogen arsenate ion HAsO42-; and finally, in basic conditions, the arsenate ion AsO43-.
|
| |
|
Arsenic is a chemical element that has the symbol As and atomic number 33. Its Atomic Mass is 74.92. Its Ionic Charge is (3-). This is a notoriously poisonous metalloid that has many allotropic forms: yellow (molecular non-metallic) and several black and gray forms (metalloids) are a few that are seen. Three metalloidal forms of arsenic with different crystal structures are found free in nature (the minerals arsenic sensu strictu and the much rarer arsenolamprite and pararsenolamprite), but it is more commonly found as arsenide and arsenate compounds. Several hundred such mineral species are known. Arsenic and its compounds are used as pesticides, herbicides, insecticides and various alloys.
The most common oxidation states for arsenic are -3 (arsenides: usually alloy-like intermetallic compounds), +3 (arsenates(III) or arsenites, and most organoarsenic compounds), and +5 (arsenates(V): the most stable inorganic arsenic oxycompounds). Arsenic also bonds readily to itself, forming, for instance, As-As pairs in the red sulfide realgar and square As43- ions in the arsenide skutterudite. In the +3 oxidation state, the stereochemistry of arsenic is affected by possession of a lone pair of electrons.
|
| |
|
|
"Online focal point for the environmental health disaster in Bangladesh
and West Bengal, India, where millions of people are drinking ground
water heavily contaminated with arsenic. Site includes infobank of news
articles, scientific papers, comprehensive links to other relevant
sites, online forum, email newsletter, and local site search." (Source:
ACIC website )
|
| |
|
As2O3: an inorganic arsenic compound; a by-product of metal smelting operations.
This small-molecule arsenic compound shows antineoplastic activity. The
mechanism of action of arsenic trioxide is not completely understood.
This agent causes damage to or degradation of the promyelocytic
leukemia protein/retinoic acid receptor-alpha (PML/RARa) fusion
protein; induces apoptosis in acute promyelocytic leukemia (APL) cells
and in many other tumor cell types; promotes cell differentiation and
suppresses cell proliferation in many different tumor cell types; and
is pro-angiogenic.
|
| |
|
 Arsenicin A, As4O3(CH2)3 is a complex molecule containing four arsenic atoms in a cage-like structure. It's found in tiny quantities in the New Caledonian marine sponge Echinochalina bargibanti and is the first poly-arsenic compound to have been isolated from nature. Its role in the marine sponge remains unknown, partly because of the lack of detailed knowledge about the chemical structure. Arsenicin A, As4O3(CH2)3 is a complex molecule containing four arsenic atoms in a cage-like structure. It's found in tiny quantities in the New Caledonian marine sponge Echinochalina bargibanti and is the first poly-arsenic compound to have been isolated from nature. Its role in the marine sponge remains unknown, partly because of the lack of detailed knowledge about the chemical structure.
Empirical formula: C3H6As4O3
|
| |
|
|
An arsenide (compound) is a compound with arsenic in oxidation state -3. An arsenide ion is an arsenic atom with three extra electrons and charge -3.
|
| |
|
|
In chemistry an arsenite is a chemical compound containing an arsenic oxoanion where arsenic has oxidation state +3. Examples of arsenites include sodium arsenite which contains a polymeric linear anion, [AsO2−]n, and silver arsenite, Ag3AsO3, which contains the trigonal, AsO33− anion. The arsenite ion is sometimes called ortho-arsenite.
|
| |
|
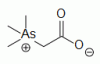 organoarsenical (CH3)3As+CH2COO-, a major arsenic containing species in marine invertebrates and fish organoarsenical (CH3)3As+CH2COO-, a major arsenic containing species in marine invertebrates and fish
|
| |
|
|
organoarsenical (CH3)3As+CH2CH2OH, a major arsenic containing species in marine invertebrates and fish
|
| |
|
FeAsS, Iron Arsenide Sulfide, an inorganic arsenic compound. This mineral is a major ore of arsenic.
For more information on the mineral Arsenopyrite see: Mineral Gallery: The Mineral Arsenopyrite
|
| |
|
|
Arsenoribofuranosides are organoarsenicals containing a pentose moiety as part of their molecular structure (see figure), which explains why they are commonly referred to arsenosugars.
Arsenosugars are major arsenic compounds in algae but are reported to be present in significant concentrations in other marine organisms such as bivalves. This class of compounds has been raising growing concern since recent reports indicating the possibility of metabolizing arsenosugars to the carcinogenic dimethylarsinic acid (DMAA) by the human body.
|
| |
|
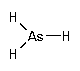 High-purity arsine is exclusively used in semiconductor manufacturing. The arsenic atom is an n-type dopant for epitaxial silicon. Arsenic is introduced in the silicon wafer by diffusion or implantation techniques. The presence of traces of arsine in the environment has been observed in gas purged from natural waters and in hot springs.
CAS Number : 7784-42-1
Other names: Arsenic hydride; Arsenic trihydride; Arsenous hydride; Hydrogen arsenide; Arsenic hydrid
|
| |
|
|
Organoarsenic compounds in which arsenic is bound onto phenyl.
The structures of the arylarsenicals that are approved as animal-food additives are shown below.
4-Hydroxy-3-nitrophenylarsonic acid (3-NHPAA) and p-arsanilic acid (p-ASA) are approved for poultry and swine. 4-Nitrophenylarsonic acid (4-NPAA) and p-ureidophenylarsonic acid (p-UPAA) are approved only for controlling blackhead disease in turkeys.
There are some compounds, such as Clark-1, Clark-2 and Adamsite, which have been developed during the first World War as warfare chemicals. Some of their degradation products (e.g. diphenyl arsinic acid) can also been found in the environment close to former production or dump sites.
|
| |
|
|
A leaching test that estimates how readily contaminants could mobilize out of a solid waste or other solid material if the material comes into contact with natural waters. The test involves placing a given mass of the solid sample in a container with a specific volume of a liquid leaching solution. The leaching solution may be water, salt buffers, acidic solutions, basic solutions, or organic solvents. The mixture is agitated for a specific amount of time (usually, hours to days). Afterwards, the mixture is filtered and the liquid (the leachate) is analyzed for contaminants, such as arsenic. A common batch leaching method is the US Environmental Protection Agency’s toxicity characteristic leaching procedure (TCLP), which is used to determine whether a solid or liquid waste has the toxicity characteristic of hazardousness (compare with leaching, leaching test, column leaching, sequential batch leaching, and serial batch leaching).
|
| |
|
|
Blackfoot disease (BFD) is a severe form of peripheral vascular disease
(PVD), in which the blood vessels in the lower limbs are severely
damaged, resulting eventually in progressive gangrene. It has been
observed in certain areas along the southwestern coast of Taiwan where the average concentration of
dissolved arsenic in the wells is 671 ± 149 µg/L with an average
As(III)/As(V) ratio of 2.6. The first symptons of the disease are the
spottet discoloration of the skin on the feet that turns from brown to
black as the disease develops until amputation of the affected
extremities, the final resort to save the BFD victims, becomes
necesaary.
|
| |
|
Carbasone was introduced in 1931 as one of the antiprotozoal organoarsenicals against infections with Trichomonas vaginalis and Entamoeba histolytica. Today it is in use as a antiparasitic food additive for poultry and swine.
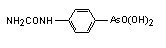 Synonyms for Carbasone: N-carbamoylarsanilic acid, [4-[aminocarbonyl-amino]phenyl] arsonic acid, Amabevan, Ameban, Amibiarson, Arsambide, Carb-O-Sep, Histocarb, Fenarsone, Leucarsone, Aminarsone, Amebarsone Synonyms for Carbasone: N-carbamoylarsanilic acid, [4-[aminocarbonyl-amino]phenyl] arsonic acid, Amabevan, Ameban, Amibiarson, Arsambide, Carb-O-Sep, Histocarb, Fenarsone, Leucarsone, Aminarsone, Amebarsone
|
| |
|
The judicious use of chelating (metal binding) agents for the removal
of toxic amounts of metal ions from living organisms. The metal ions
are sequestered by the chelating agents and are rendered harmless or
excreted. Chelating agents such as 2,3-dimercaptopropan-1-ol,
ethylenediaminetetraacetic acid, desferrioxamine and D-penicillamine
have been used effectively in chelation therapy for arsenic, lead, iron
and copper, respectively.
|
| |
|
|
Occurs in solutions and refers to a minor or trace element (such as arsenic) adsorbing onto or absorbing within the developing or fresh precipitates of other chemical species. Although sometimes difficult to distinguish, sorption involves the incorporation of contaminants onto or within preexisting solids (sorbents), whereas coprecipitation occurs as or shortly after the host solids precipitate from solution, such as arsenic coprecipitating with iron (oxy)(hydr)oxides in acid mine drainage. Coprecipitation might also involve arsenic-bearing colloids or other fine-grained particles becoming trapped (absorbed) in the interiors of precipitating compounds (compare with precipitation and sorption).
|
| |
|
A small-molecule organic arsenical with potential antineoplastic activity. Although the exact mechanism of action is unclear, darinaparsin, a highly toxic metabolic intermediate of inorganic arsenicals (iAs) that occurs in vivo, appears to generate volatile cytotoxic arsenic compounds when glutathione (GSH) concentrations are low. The arsenic compounds generated from darinaparsin disrupt mitochondrial bioenergetics, producing reactive oxygen species (ROS) and inducing ROS-mediated tumor cell apoptosis; in addition, this agent or its byproducts may initiate cell death by interrupting the G2/M phase of the cell cycle and may exhibit antiangiogenic effects. Compared to inorganic arsenic compounds such as arsenic trioxide (As2O3), darinaparsin appears to exhibit a wide therapeutic window.
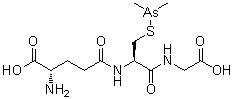
Name: Darinaparsin
IUPAC/Chemical name: (S)-2-amino-5-(((R)-1-((carboxymethyl)amino)-3-((dimethylarsino)thio)-1-oxopropan-2-yl)amino)-5-oxopentanoic acid
CAS#: 69819-86-9
Chemical Formula: C12H22AsN3O6S
Exact Mass: 411.04453
Molecular Weight: 411.31
Elemental Analysis: C, 35.04; H, 5.39; As, 18.22; N, 10.22; O, 23.34; S, 7.80
|
| |
|
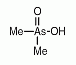 Dimethylarsinic acid (DMAV) is an organoarsenic compound that has been identified as one of the main metabolites found in urine of humans (and animals) after ingestion of inorganic arsenic compounds. Dimethylarsinic acid (DMAV) is an organoarsenic compound that has been identified as one of the main metabolites found in urine of humans (and animals) after ingestion of inorganic arsenic compounds.
|
| |
|
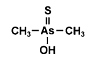 Dimethylarsinothioic acid (DMAS) also called dimethylthioarsinic acid (DMTAV) is an organoarsenic compound that has recently
been identified as human metabolite after exposure toward both the human
carcinogen inorganic arsenic and arsenosugars, which are the major
arsenical constituents of marine algae. It was earlier already identified as a metabolite in sheep's urine after ingestion of seaweed containing arsenosugars. Dimethylarsinothioic acid (DMAS) also called dimethylthioarsinic acid (DMTAV) is an organoarsenic compound that has recently
been identified as human metabolite after exposure toward both the human
carcinogen inorganic arsenic and arsenosugars, which are the major
arsenical constituents of marine algae. It was earlier already identified as a metabolite in sheep's urine after ingestion of seaweed containing arsenosugars.
|
| |
|
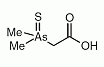 Dimethylarsinothioyl acetic acid (DMAAS) is an organoarsenic compond that has been identified as a minor metabolite in sheep's urine after ingestion of seweed containing arsenosugars. Dimethylarsinothioyl acetic acid (DMAAS) is an organoarsenic compond that has been identified as a minor metabolite in sheep's urine after ingestion of seweed containing arsenosugars.
|
| |
|
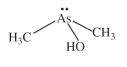 An organic trivalent arsenical with the composition of (CH3)2As(OH), abbreviated DMA(III), see Figure (compare with dimethylarsinic acid, monomethylarsonic acid, and monomethylarsonous An organic trivalent arsenical with the composition of (CH3)2As(OH), abbreviated DMA(III), see Figure (compare with dimethylarsinic acid, monomethylarsonic acid, and monomethylarsonous
acid).
|
| |
|
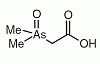 Dimethylarsinoyl acetic acid (DMAA) is an organoarsenic compound found in sheep's urine as a minor metabolite after ingestion of seaweed containing arsenosugars. Dimethylarsinoyl acetic acid (DMAA) is an organoarsenic compound found in sheep's urine as a minor metabolite after ingestion of seaweed containing arsenosugars.
|
| |
|
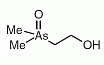 Dimethylarsinoylethanol (DMAE) was found in sheep's urine as a metabolite after seaweed consumption containing arsenosugars as the main arsenic compounds.
|
| |
|
Dimethylarsonyldimethylarsinic acid has been identified as one of the organoarsenic species present 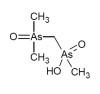 in some rice grain samples. It is present in some rice samples both as oxide and as mono- and di-thiolated compound. The thiolated forms are highly sensitive to the extraction conditions, especially the application of heat and the use of hydrogen peroxide.
|
| |
|
|
Diphenylarsinic acid (DPAA) is a degradation product of organoarsenic warfare chemicals in the environment. Due to the breakdown of some buried storage vessels or chemical warheads organoarsenic warfare chemicals can be released and DPAA can contaminate underground or well water.
|
| |
|
|
Diphenylchloroarsine (DA) is an organoarsenic compound developed near the conclusion of World War I. The chemical warfare compound is solid when pure, and has been used as an aerosol dispersed by thermal grenades or smoke generators. Its effect are: severe irritation of the eyes, nose, and throat. If the agent is inhaled for 1-2 minutes, tightness of the chest and headache are experienced. The headache develops into general nausea, which can result in vomiting in approximately three minutes. Under concentrations expected to occur under combat conditions, fatalities are not expected; however, these compounds can be fatal at higher concentrations.
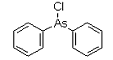 DA was used by German troops in 1917 and was considered a significant development because it penetrated the activated carbon gas mask filters deployed in World War I. Its irritant behavior was considered more important than its lethality. DA was used in combination with phosgene and diphosgene; DA caused victims to remove their masks to sneeze, cough, or vomit, rendering them vulnerable to the toxic effects of the other agents. DA alone saw some use as a riot-control agent up to the 1930s.
Names: diphenylarsinous chloride, diphenylchloroarsine, Clark-1
Molecular formula: C12H10AsCl
CAS Registry Number: 712-48-1
|
| |
|
|
Diphenylarsinous cyanide (DC) is an organoarsenic compound developed near the conclusion of World War I. The chemical warfare compound is solid when pure, and has been used as an aerosol dispersed by thermal grenades or smoke generators. Its effect are: severe irritation of the eyes, nose, and throat. If the agent is inhaled for 1-2 minutes, tightness of the chest and headache are experienced. The headache develops into general nausea, which can result in vomiting in approximately three minutes. Under concentrations expected to occur under combat conditions, fatalities are not expected; however, these compounds can be fatal at higher concentrations.
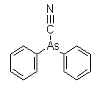 DC was used by the Germans in 1918. It was intended to combine the vomiting potential of DA with the lethality of cyanide. However, DC did not prove particularly lethal in tests. DC was a standard agent (Red No. 1) in the arsenal of the Japanese Imperial Forces between 1931 and 1945.
Names: DC, Diphenylarsinous cyanide, Clark II, diphenylcyanoarsine
Molecular formula: C13H10AsN
CAS Registry Number: 23525-22-6
|
| |
|
|
One of the 103 known chemical substances
that cannot be broken down further without changing its chemical properties.
Some examples include arsenic, cadmium, lead and mercury.
|
| |
|
|
Sometimes referred to in short as inorganic arsenic, inorganic
arsenic compound contain arsenic (As) and at least one other element,
but no carbon (C). Inorganic arsenic exists in four main chemical forms
known as valency or oxidation states. Valency is a measure of the
ability of a compound to combine with other elements, such as hydrogen.
The dominant forms are:
- Arsenite, with a valency of 3, also referred to as trivalent
arsenic (As (III), As+3), and
- Arsenate, with a valency of 5, also referred to as pentavalent
arsenic (As (V), As+5
Inorganic arsenic (compounds) are mainly of geological origin and can be found
in groundwater used as drinking water in certain parts of the world.
|
| |
|
|
Classified as a blister agent (vesicant), Lewisite is named after the American military scientist, W. Lee Lewis, who produced this organoarsenic compound as a prototype chemical warfare (CW) agent in 1918. Because it was developed so late in World War I, Lewisite was never used in that conflict. The only military use of Lewisite known probably occurred in China during the Sino-Japanese conflict (ca. 1937-1942). Unlike mustard, which has delayed onset of clinical symptoms, the extreme irritation to eyes and skin begin almost immediately, with redness and blisters forming hours later. Significant exposure to Lewisite can cause blindness. Because of the rapid onset of pain, however, most exposures to Lewisite will result in less damage to the eyes as victims will attempt to avoid further contact by closing eyelids and avoiding the area. Lewisite, in its pure form, is an oily, colorless liquid, with no detectable odor. However, some have described impure batches of Lewisite as being of amber or dark brown color and having an odor of geraniums.
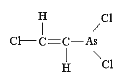 Depending on environmental conditions, Lewisite is a semi-persistent agent, and can penetrate a variety of rubber products, including those used in protective garments. Liquid at low temperatures (well below freezing), Lewisite was sometimes employed to mix with mustard to keep both CW agents solvent for use in winter conditions. Concentrations of Lewisite that can cause injury and death closely resemble those of mustard agent (also a vesicant). The median lethal concentration (LCt50) for Lewisite is about 1.5 grams-min/m3 for inhalation, and the median lethal dose (LD50) on the skin is estimated at 30mg/kg, or about 2.5 grams for an adult male of 180 pounds. The chemical formulation of Lewisite is relatively simple, and many countries, including those in the developing world, are capable of producing it in militarily significant quantities. |
| |
|
|
"The London Arsenic Group brings together expertise from the fields
of sedimentary geochemistry, hydrochemistry, environmental mineralogy
and analytical geochemistry. We seek to understand the source,
mobility, and fate, of arsenic in the environment. We exist to bring a
multi-disciplinary approach to this issue and provide a focus for
exchange of views."
(Source: LAG website )
|
| |
|
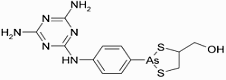 Melarsoprol (INN) is a medicinal drug used in the treatment of trypanosomiasis, such as Chagas disease and West African sleeping sickness, the former caused by Trypanosoma cruzi and the latter by Trypanosoma brucei gambiense. It is also sold under the trade names “Mel B” and “Melarsen Oxide-BAL.” Melarsoprol (INN) is a medicinal drug used in the treatment of trypanosomiasis, such as Chagas disease and West African sleeping sickness, the former caused by Trypanosoma cruzi and the latter by Trypanosoma brucei gambiense. It is also sold under the trade names “Mel B” and “Melarsen Oxide-BAL.”
Being a toxic organic compound of arsenic, melarsoprol is a highly dangerous treatment which is only administered by injection under the supervision of a physician, as it can produce similar effects as arsenic poisoning. Negative side effects caused by Mlarsoprol include convulsions, fever, loss of consciousness, rashes, bloody stools, nausea, and vomiting. It is fatal in and of itself in around 8% of cases.
IUPAC name: (2-(4-(4,6-diamino-1,3,5-triazin-2-ylamino)phenyl)-1,3,2-dithiarsolan-4-yl)methanol
Formula: C12H15AsN6OS2
Mol. mass: 398.341 g/mol
CAS number: 494-79-1
|
| |
|
|
Together with the metals and nonmetals, the metalloids (in Greek metallon = metal and eidos = sort - also called semimetals) form one of the three categories of chemical elements as classified by ionization and bonding properties. They have properties intermediate between those of metals and nonmetals. There is no unique way of distinguishing a metalloid from a true metal but the most common is that metalloids are usually semiconductors rather than conductors.
The known metalloids (and their atomic symbols) are: Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te) and Polonium (Po)
|
| |
|
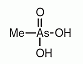 Methylarsonic acid (MAV) is an organoarsenic compound found as a minor metabolite in urine of humans and animals after ingestion of inorganic arsenic compounds. Methylarsonic acid (MAV) is an organoarsenic compound found as a minor metabolite in urine of humans and animals after ingestion of inorganic arsenic compounds.
|
| |
|
|
A chemical species containing pentavalent arsenic, sulfide, and methyl groups, as examples: (CH3)AsO2S2−, (CH3)AsOS22−, (CH3)2AsOS−, and (CH3)2AsS2− (compare with thioarsenic, thioarsenate, and thioarsenite).
|
| |
|
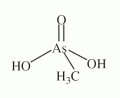 An organic pentavalent arsenical with the composition of (CH3)AsO(OH)2, abbreviated MMA(V), see Figure (compare with dimethylarsinous acid, dimethylarsinic acid, and monomethylarsonous acid). An organic pentavalent arsenical with the composition of (CH3)AsO(OH)2, abbreviated MMA(V), see Figure (compare with dimethylarsinous acid, dimethylarsinic acid, and monomethylarsonous acid).
|
| |
|
Arsenic compound (4-nitrophenylarsonic acid, 4-NPAA) used as animal
feed additive for the prevention of blackhead (Histomoniasis) in
turkeys.
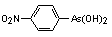
|
| |
|
|
Arsenic compounds containing carbon. They are mainly found in sea-living
organisms, although some of these compounds have also been found in species
living on land.
|
| |
|
|
Compound with at least one covalent bond (sigma or pi) between a carbon atom and a metal atom. This term excludes other metal-organic complexes in which the metal is usually bound to nitrogen, oxygen, or phosphorus. It also excludes metaloids such as arsenic and selenium which can form covalent bonds with alkyl and aryl groups.
|
| |
|
Organoarsenical (3-nitro-4-hydroxyphenylarsonic acis, 3-NHPAA)
used as s feed additive for broiler chickens in order to
control coccidial intestinal parasites, thereby improving feeding
efficiency.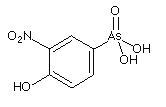
|
| |
|
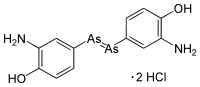 Arsphenamine, also known as Salvarsan and 606, is a drug containing arsenic that was used to treat syphilis and trypanosomiasis. The organoarsenic compound was the first modern chemotherapeutic agent. Arsphenamine was marketed under the trade name Salvarsan in 1910. It was also called 606, because it was the 606th compound synthesized for testing. Salvarsan was the first organic anti-syphillitic, and a great improvement over the inorganic mercury compounds that had been used previously. A more soluble (but slightly less effective) arsenical compound, Neosalvarsan, (neoarsphenamine), became available in 1912. These arsenical compounds came with considerable risk of side effects, and they were supplanted as treatments for syphilis in the 1940s by penicillin. Arsphenamine, also known as Salvarsan and 606, is a drug containing arsenic that was used to treat syphilis and trypanosomiasis. The organoarsenic compound was the first modern chemotherapeutic agent. Arsphenamine was marketed under the trade name Salvarsan in 1910. It was also called 606, because it was the 606th compound synthesized for testing. Salvarsan was the first organic anti-syphillitic, and a great improvement over the inorganic mercury compounds that had been used previously. A more soluble (but slightly less effective) arsenical compound, Neosalvarsan, (neoarsphenamine), became available in 1912. These arsenical compounds came with considerable risk of side effects, and they were supplanted as treatments for syphilis in the 1940s by penicillin.
Source: Wikipedia
|
| |
|
|
An aqueous species, such as H2AsO3S− and H2AsS2O2−, that contains pentavalent arsenic and sulfide (compare with arsenate, thioarsenic, thioarsenite, and methylthioarsenate).
|
| |
|
|
An aqueous species, such as HAs3S62− and H2AsO3S−, that contains sulfide and arsenic, either as arsenide (As3−), As3+, or As5+ (compare with arsenosulfide, methylthioarsenate, thioarsenate, and thioarsenite).
|
| |
|
|
An aqueous species, such as H2As3S6−, that contains trivalent arsenic and sulfide (compare with arsenite, thioarsenic, thioarsenate, and methylthioarsenate).
|
| |
|