|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
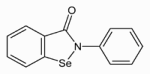 Ebselen or 2-phenyl-1, 2-benzisoselenazol-3(2H)-one (also called PZ 51 or DR3305), is a mimic of glutathione peroxidase and can also react with peroxynitrite. It is being investigated as a possible treatment for reperfusion injury and stroke, as well as tinnitus. Ebselen or 2-phenyl-1, 2-benzisoselenazol-3(2H)-one (also called PZ 51 or DR3305), is a mimic of glutathione peroxidase and can also react with peroxynitrite. It is being investigated as a possible treatment for reperfusion injury and stroke, as well as tinnitus.
Ebselen is a potent scavenger of hydrogen peroxide as well as hydroperoxides including membrane bound phospholipid and cholesterylester hydroperoxides.
Chemical formula: C13H9NOSe
Molecular mass: 274.17666
CAS-Number: 60940-34-3
|
| |
|
|
A single scribed groove on a diffraction grating.
|
| |
|
|
A diffraction grating designed to operate with incident and diffracted
light at an angle greater than 45° from the grating normal.
|
| |
|
|
Ecotoxicology is a research field that explores how exposure to a toxicant negatively affects single organisms, populations, communities, and ecosystems.
|
| |
|
|
Energy-dispersive X-ray microanalysis (sometimes also known as EDS: Energy-dispersive X-ray Spectroscopy and EDAX.) |
| |
|
|
The material that is coming from a chromatographic separation. Can also be the waste outfall from industries. Also is the term for sewage (sewage effluent).
|
| |
|
|
The electric sector is a device constructed of curved, parallel metal plates that creates an electrostatic field perpendicular to the ion path. The sector (or analyzer) selects and focuses ions of the same kinetic energy. The electric sector does not separate ions according to mass or charge, and therefore is always used in conjunction with a magnetic analyzer, often in a double-focusing mass spectrometer. The electric sector is also sometimes called an electrostatic sector or electrostatic analyzer.
|
| |
|
|
- the distribution of solution ions at a charged surface.
|
| |
|
|
Electro-osmosis is the motion of a liquid through a membrane (or plug or capillary) as a consequence of the application of an electric field across the membrane
|
| |
|
|
The application of electrochemical cells and electrochemical techniques for
chemical analysis. The analyte is dissolved in the electrolyte of the cell, and
one can perform either "qualitative" analysis (determination of the type of
constituents present) or "quantitative" analysis (determination of the amount of
a given constituent).
|
| |
|
|
Electrodeposition (which includes electro-crystallization) is the deposition of dissolved or suspended material by an electric field on an electrode.
|
| |
|
|
- a substance that separates into ions when in solution and therefore becomes capable of conducting electricity; an ionic solute.
|
| |
|
Electron attachment is a process in which an electron of thermal energy is added to an atom or molecule (M) to form a stable ion (M- ). The molecule must have a positive electron affinity (for example, be electrophoric). Thermal electrons are required so as not to cause dissociation of the molecular ion.
  
|
| |
|
A device that responds to decreases in an electrical current as the analyte passes through a plasma.
In ECD, a detector ionizes solutes by collision with metastable carrier-gas molecules produced by ß-emission from a radioactive source such as 63Ni. The electron-capture detector is one of the most sensitive detectors, and it responds strongly to halogenated solutes and others with high electroncapture cross-sections.
|
| |
|
|
Electron impact ionization or as currently preferred electron ionization
represents the classical method of ion generation in mass spectrometry. The analyte is
introduced into the evacuated ion source (<10-6 mbar) and subsequently ionized by collisions with energetic electrons (routine 70 eV) generated by thermal emission from a
heated filament.
|
| |
|
An electron multiplier is a detection device inside the vacuum of the mass spectrometer that converts the arrivals of ions at its front dynodes into a detectable, amplified electron current at the back lead of the device. The overall gain (signal out/signal in) can be as high as 104–108. Positive ions exiting from the mass analyzer impact the first dynode surface, and the impact causes the release of several electrons, which are then accelerated through a potential to the next electrode. There, each electron impact causes the release of several secondary electrons, which are accelerated into the next dynode for a repetition of the impact–release process. A cascade of electrons is produced, generating a current that is further amplified and then sampled by an analog-to-digital converter to be recorded by the data system.
  
|
| |
|
|
A form of molecular spectroscopy concerned with microwave-induced transitions between magnetic energy levels of electrons having a net spin and orbital angular momentum. The spectrum is normally obtained by magnetic field scanning. Also known as electron spin resonance (ESR) spectroscopy or electron magnetic resonance (EMR) spectroscopy.
The frequency (ν) of the oscillating magnetic field to induce transitions between the magnetic energy levels of electrons is measured in gigahertz (GHz) or megahertz (MHz). The following band designations are used: L (1.1 GHz), S (3.0 GHz) , X (9.5 GHz), K (22.0 GHz) and Q (35.0 GHz). The static magnetic field at which the EPR spectrometer operates is measured by the magnetic flux density (B) and its recommended unit is the tesla (T). In the absence of nuclear hyperfine interactions, B and are related by : h ν = g µBB where h is the Planck constant, µB is the Bohr magneton, and the dimensionless scalar g is called the g-factor. When the paramagnetic species exhibits an anisotropy, the spatial dependency of the g-factor is represented by a 3x3 matrix. The interaction energy between the electron spin and a magnetic nucleus is characterized by the hyperfine coupling constant A. When the paramagnetic species has anisotropy, the hyperfine coupling is expressed by a 3x3 matrix called a hyperfine coupling matrix. Hyperfine interaction usually results in splitting of lines in an EPR spectrum. The nuclear species giving rise to the hyperfine interaction should be explicitly stated, e.g."the hyperfine splitting due to 65Cu". When additional hyperfine splittings due to other nuclearspecies are resolved ("superhyperfine"), the nomenclature should include the designation of the nucleus, and the isotopic number
|
| |
|
|
EPMA (EMPA) is an analytical technique used to determine the chemical nature of extremely small samples by forming the X-ray spectrum of the sample through excitation by a finely focused electron beam.
|
| |
|
|
- the bulk flow of liquid in CE. It is the motion of a liquid relative to a fixed charged surface caused by an electric field.
|
| |
|
|
- the data output from CE; a plot of detector response against migration time.
|
| |
|
|
Electrophoresis is based on the migration of charged molecules in solution in response to an electric field. Their rate of migration depends on the strength of the field; on the nett charge, size and shape of the molecules and also on the ionic strength, viscosity and temperature of the medium in which the molecules are moving. As an analytical tool, electrophoresis is simple, rapid and highly sensitive. It is used analytically to study the properties of a single charged species, and as a separation technique.
|
| |
|
|
- the factor that determines the rate at which a given ionic solute may move by electrophoresis.
|
| |
|
|
A mass spectrometric technique for liquid samples that involves preparing electrically charged droplets from analyte molecules dissolved in a solvent. The electrically charged droplets enter a vacuum chamber where the solvent is evaporated. Evaporation of solvent reduces the droplet size, thereby increasing the coulombic repulsion within the droplet. As the charged droplets get smaller, the excess charge within them causes them to disintegrate and release analyte molecules. The volatilized analyte molecules are then analyzed by mass spectrometry.
|
| |
|
|
An electrospray needle is the narrow bore tube from which highly charged droplets are emitted in the electrospray proces.
|
| |
|
A chemical bond of two atoms or molecules by an electrostatic force (like static electricity) caused by one or more electrons moving from one atom to the other.
|
| |
|
An electrothermal atomizer is a device which is heated, to the temperature required for analyte atomization, by the passage of electrical current through its body. This technique has largely been developed for use in atomic absorption spectrometry for which the terms electrothermal atomic absorption spectrometry, electrothermal AAS and the abbreviation ETAAS are defined. It has also been applied in atomic emission and atomic fluorescence spectrometry, with appropriate analogous terms, such as electrothermal atomic emission spectrometry (ETAES) and electrothermal atomic fluorescence spectrometry (ETAFS), being defined.
source: IUPAC Analytical Compendium, Chapter 10
|
| |
|
An electrothermal vaporizer (ETV) is a device which is heated, to the temperature required for analyte vaporization, by the passage of electrical current through its body. The ETV is used as a sample introduction device for liquid and solid samples into a spectroscopic excitation or ionization source such as a flame or plasma discharge. The combined technique allows for the independent control of the vaporization parameters and the atomization/ionization parameters. Combined techniques often used are: ETV-ICP-AES, ETV-ICP-MS, ETV-MIP-AES
|
| |
|
|
One of the 103 known chemical substances
that cannot be broken down further without changing its chemical properties.
Some examples include arsenic, cadmium, lead and mercury.
|
| |
|
Elemental analysis is a process used to evaluate a variety of organic materials for carbon, hydrogen, nitrogen, oxygen, and sulfor content.
|
| |
|
|
In elemental mass spectrometry, a technique used mostly for inorganic
materials, the elemental composition of a sample is determined
rather
than the structural identities of its chemical constituents.
Elemental
mass spectrometry provides quantitative information about the
concentrations
of those elements. The ion source used in elemental MS is
ordinarily
an atmospheric-pressure discharge such as the inductively
coupled plasma
(ICP) or a moderate-power device such as the glow-discharge
source.
In either case, the decomposition of the sample into its
constituent
atoms and ionization of those atoms occurs in a specially
designed source.
The resulting atomic-ion beam is then separated or sorted by a
mass
spectrometer and the signal as a function of m/z used to
determine the
sample composition.
|
| |
|
Hg. Mercury in its elemental (pure) form,
that is, as a metal; hence the synonym metallic mercury. A shiny, silver-gray
metal that is a liquid at room temperature.
Elemental mercury has many uses and is for instance found in thermometers,
barometers, dental amalgams, batteries, fluorescent lights and some electrical
switches. It is rarely found in nature.
|
| |
|
|
Combination of mobile phase and solute exiting the column; also called effluent.
|
| |
|
|
The liquid or gas entering the chromatographic bed and used to effect a separation by elution.
|
| |
|
|
A procedure in which the mobile phase is continuously passed through or along the chromatographic bed and the sample is fed into the system as a finite slug
|
| |
|
A process that creates a stable mixture of two liquids that normally would not mix together (such as oil and water) by forcing one to disperse in the other as droplets.
|
| |
|
|
One of two indistinguishable forms of a compound that differ only in the orientation in space; a stereoisomer.
|
| |
|
Originating internally. In the description of metal ion coordination in
metalloproteins, endogenous refers to internal, or protein-derived,
ligands.
|
| |
|
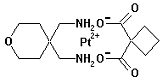 Enloplatin is a platinum metallodrug developed for cancer therapy. Enloplatin is a platinum metallodrug developed for cancer therapy.
Chemical Synonyms (1,1-cyclobutanedicarboxylato(2-)-O,O')(tetrahydro-4H-pyran- 4,4-dimethanamine-N,N') platinum(II)
CL 287110, CL-287110
CAS Registry No. 111523-41-2
|
| |
|
|
Enteral nutrition refers to any method of feeding that uses the
gastrointestinal (GI) tract to deliver nutrition and calories. It can
include a normal oral diet, the use of liquid supplements or delivery by
use of a tube (tube feeding).
|
| |
|
|
Soil, water, air, biota (plants and animals), or any other parts of the environment that can contain contaminants.
|
| |
|
|
Continuous or repeated measurement of agents in the environment to evaluate environmetal exposure and possible damage by comparison with appropriate reference values based on knowlege of the probable relationship between ambient exposure and resultant adverse effects.
|
| |
|
The digestion of a peptide, protein, or mixtures of peptides and/or proteins with an enzyme such as trypsin, chymotrypsin, pepsin, endoproteinase Glu-C, endoproteinase Lys-C and endoproteinase Asp-N. This is a key step in many proteomics analyses.
|
| |
|
A macromolecule that functions as a biocatalyst by increasing the
reaction rate, frequently containing or requiring one or more metal
ions. In general, an enzyme catalyzes only one reaction type (reaction
specificity) and operates on only a narrow range of substrates
(substrate specificity). Substrate molecules are attacked at the same
site (regiospecificity) and only one or preferentially one of the
enantiomers of chiral substrate or of racemic mixtures is attacked
(enantiospecificity).
|
| |
|
|
Process whereby an enzyme is synthesized in response to the presence of a specific substance or to other agents such as heat or a metal.
|
| |
|
Proteins that catalyze biochemical reactions by causing or speeding up reactions without being changed in the process themselves.
|
| |
|
|
Abbreviation for "Extractable Organohalogens".
The fraction of AOX which is extractable by a non-polar organic
solvent. This fraction contains the relatively lipophilic
(fat-soluble) organic compounds. EOX gives a better indication of
the amount of organic halogens susceptible to lipophilic
absorption. It often represents about one tenth of the AOX
measured.
|
| |
|
|
United States Environmental Protection Agency.
|
| |
|
|
The study of the causes, distributions, and management of diseases within human populations.
|
| |
|
Electrothermal atomic absorption spectrometry : A type of atomic absorption spectrometry where the sample is atomised using a probe which is rapidly heated by passing a current through it. The probe often is either a graphite tube or a tungsten coil.
|
| |
|
|
introduction of an ethyl group into a compound
see also: alkylation
|
| |
|
|
C2H5Hg+. Ethylmercury is a cation that forms organic mercury
compounds such as ethylmercury chloride and ethylmercury urea. Thimerosal is
also an ethylmercury salt: sodium ethylmercuric thiosalicylate. The term
'ethylmercury' is sometimes used as a generic term to describe ethylmercury
compounds.
|
| |
|
|
The Scientific Committee on Toxicity, Ecotoxicity and the Environment (CSTEE)
was created by the European Commission to address "scientific and
technical questions relating to examination of the toxicity and
ecotoxicity of chemical, biochemical and biological compounds whose use
may have harmful consequences for human health and the environment."
(Source: CSTEE website )
|
| |
|
|
"Following a series of food scares in the 1990s (eg BSE, dioxins…)
which undermined consumer confidence in the safety of the food chain,
the European Union concluded that it needed to establish a new
scientific body charged with providing independent and objective advice
on food safety issues associated with the food chain. Its primary
objective as set out in the White Paper on Food Safety would be to:
“…contribute to a high level of consumer health protection in the area
of food safety, through which consumer confidence can be restored and
maintained.” The result was the European Food Safety Authority (EFSA).
Set
up provisionally in Brussels in 2002, EFSA provides independent
scientific advice on all matters linked to food and feed safety -
including animal health and welfare and plant protection - and provides
scientific advice on nutrition in relation to Community legislation.
The Authority communicates to the public in an open and transparent way
on all matters within its remit. EFSA’s risk assessments provide risk
managers (consisting of EU institutions with political accountability,
i.e. European Commission, European Parliament and Council) with a sound
scientific basis for defining policy driven legislative or regulatory
measures required to ensure a high level of consumer protection with
regards to food safety." (Source: EFSA website )
|
| |
|
An even-electron ion contains no unpaired electrons; for example, CH3+.
  
|
| |
|
|
state of a system with energy higher than that of the ground state
|
| |
|
|
Originating externally. In the context of metalloprotein ligands,
exogenous describes ligands added from an external source, such as CO
or O2.
|
| |
|
|
Quantity defining an interval
about the result of a measurement that may be expected to encompass a
large fraction of the distribution of values that could reasonably be
attributed to the measurand.
|
| |
|
Contact with a substance by swallowing, breathing, or touching the skin
or eyes. Exposure may be short-term [acute exposure],
of intermediate duration, or long-term [chronic
exposure].
|
| |
|
The process of finding out how people come into contact with a hazardous
substance, how often and for how long they are in contact with the substance,
and how much of the substance they are in contact with.
Source: ATSDR Glossary of Terms
|
| |
|
The route a substance takes from its source (where it began) to its end
point (where it ends), and how people can come into contact with (or get
exposed to) it. An exposure pathway has five parts: a source of contamination
(such as an abandoned business); an
environmental
media and transport mechanism (such as movement through groundwater);
a point of exposure (such as a private
well); a route of exposure (eating, drinking,
breathing, or touching), and a receptor
population (people potentially or actually exposed). When all five
parts are present, the exposure pathway is termed a completed exposure
pathway.
Source: ATSDR Glossary of Terms
|
| |
|
A method of estimating the amount of people's past exposure to hazardous
substances. Computer and approximation methods are used when past information
is limited, not available, or missing.
Source: ATSDR Glossary of Terms
|
| |
|
|
Part of an X-ray absorption spectrum that is used to identify the coordination of atoms, estimate bond lengths, and determine the adsorption complexes on the surfaces of adsorbents. EXAFS spectra may provide useful information on the speciation (valence state), surface complexes, and the coordination of the target atom (compare with X-ray absorption spectroscopy (XAS), X-ray absorption near edge structure (XANES) spectra, and X-ray absorption fine structure spectroscopy (XAFS)).
|
| |
|
|
"The EXTension TOXicology NETwork (EXTOXNET) is an effort of
University of California, Davis, Oregon State University, Michigan
State University, Cornell University, and the University of Idaho.
Some
of the goals of EXTOXNET are to stimulate dialog on toxicology issues,
develop and make available information relevant to extension
toxicology, and facilitate the exchange of toxicology-related
information in electronic form, accessible to all with access to the
Internet.
The EXTOXNET InfoBase is accessible via the World Wide Web (WWW)."
(Source: EXTOXNET website )
|
| |
|
|
A measurand, usually identical with the analyte, which is analysed separately from the sample.
|
| |
|
|
An extracted ion chromatogram is a plot of the signal intensity at one or more selected m/z values in a series of mass spectra that are recorded as a function of chromatographic retention tim.
|
| |
|
|
An extracted ion profile is the plot of signal intensity observed at one or more selected m/z
values in a series of mass spectra that are recorded as a function of
time. Examples are extracted ion chromatogram, extracted ion
electropherogram, flow injection analysis or any other time-dependent
samplin.
|
| |
|
|
The removal of a soluble material from a solid mixture by means of a solvent or the removal of one or more components from a liquid mixture by use of a solvent with which the liquid is immiscible or nearly so.
|
| |
|
|
Amount of substance extracted from a source divided by the total contained within the source.
|
| |
|