|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
|
A statistical test of significance in which the
difference between two variances is tested. A variance is the square of
a standard deviation. The F-test is often used to compare the
imprecision of two analytical methods. The hypothesis being tested
(called a null hypothesis) is that there is no difference between the
two variances. If the calculated F-value is greater than the critical
value which is obtained from a statistics table, then the null
hypothesis is rejected. This means that a difference exists and that the
difference is statistically significant, or real. If the calculated
F-value is less than the critical value, the null hypothesis cannot be
rejected, therefore, there is no difference between the two variances
being tested, and the difference is not statistically significant.
|
| |
|
|
In flame atomic absorption spectrometry,
the sample is nebulized and sucked into a burner, so that analyte
elements can be atomized in a flame of a fuel gas, such as acetylene,
and an oxidation gas, usually air. The burner is positioned in such a
way that the flame region with the maximum atom concentration is in the
ray path of the spectrometer. These atoms are able to absorb element-specific radiation. To this end,
an element-specific lamp with a hollow cathode made of the element to be
investigated is introduced into the ray path of an atomic absorption
spectrometer with the atomization device and a detector. Depending on
the concentration of the element to be determined in the sample, some of
the radiation intensity of the hollow-cathode lamp is absorbed by the
atoms formed. The detection system (often a photomultiplier or photodiode) measures the intensity of the
non-attenuated radiation and the radiation after leaving the atomization
device during the supply of a sample solution. The element
concentration in the sample can then be calculated from the difference in the
two intensities.
|
| |
|
|
A hollow collector, open at one end and closed at the other, used to collect and measure, beams of ions.
|
| |
|
|
Fast atom bombardment is the formation of ions by the interaction of a
focused beam of neutral atoms with a translational energy of several keV
impinging on a sample that is typically dissolved in a solvent matrix
|
| |
|
A mass spectrometric technique that is used for the analysis of a wide
range of biomolecules, such as glycoalkaloids, glycoproteins,
polysaccharides, and peptides. Positive and negative fast atom
bombardment spectra are recorded on a mass spectrometer fitted with an
atom gun with xenon as the customary beam. The mass spectra obtained
contain molecular weight recognition as well as sequence information.
|
| |
|
|
FPLC: a termed coined to cover the specific use of HPLC for separating proteins. Generally, glass columns, moderate pressure, and spherical microbeads are used for FPLC.
|
| |
|
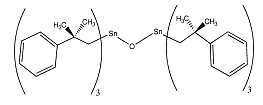 Fenbutatin
oxide is an organotin compound used as an insecticide. It acts as
contact and stomach acaricides for the control of various mites in a
variety of fruit, vegetable and field crops. Fenbutatin
oxide is an organotin compound used as an insecticide. It acts as
contact and stomach acaricides for the control of various mites in a
variety of fruit, vegetable and field crops.
IUPAC name: bis[tris(2-methyl-2-phenylpropyl)tin] oxide
CAS name: hexakis(2-methyl-2-phenylpropyl)distannoxane
Molecular formula: C60H78OSn2
Molecular weight: 1053
|
| |
|
A protein containing more than one iron and acid-labile sulfur, that
displays electron-transferactivity but not classical enzyme function.
|
| |
|
|
An iron(III) porphyrin coordination complex.
|
| |
|
|
An iron storage protein consisting of a shell of 24 protein subunits, encapsulating up to 4500 iron atoms in the form of a hydrated iron(III) oxide.
|
| |
|
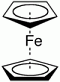 Ferrocene is the organometallic compound with the formula Fe(C5H5)2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds. Ferrocene is the organometallic compound with the formula Fe(C5H5)2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds.
|
| |
|
|
An iron(II) porphyrin coordination complex.
|
| |
|
|
A sulfate salt of mineral iron formulated for oral administration and
used as a dietary supplement, ferrous sulfate is absorbed in the
stomach and small intestine and combines with apoferritin to form
ferritin, which is stored in the liver, spleen, red bone marrow, and
intestinal mucosa. Important in transport of oxygen by hemoglobin to
the tissues, iron is also found in myoglobin, transferrin, and
ferritin, and is a component of many enzymes such as catalase,
peroxidase, and cytochromes.
|
| |
|
|
A synthetic ultrasmall superparamagnetic iron oxide composed of
dextran-coated iron oxide nanoparticles (also known as 'ultrasmall
particulate iron oxides' or USPIO). Ferumoxtran-10, which accumulates
in non-cancerous lymphatic tissue, is used as a molecular resonance
imaging (MRI) contrast agent.
|
| |
|
|
A superparamagnetic iron oxide nanoparticle coated with a low molecular
weight semi-synthetic carbohydrate, polyglucose sorbitol carboxymethyl
ether, with potential anti-anemic and imaging properties. After
intravenous administration, ferumoxytol replaces iron stores with fewer
side effects compared to the use of oral iron. In addition, this agent
generates T1 relaxation, producing a magnetic field and enhancing T2
relaxation, thereby darkening contrast media-containing structures in
magnetic resonance imaging (MRI). Due to small particle size,
ferumoxytol remains in the intravasculature for a prolonged period and
so may be used as a blood pool agent.
|
| |
|
|
Field flow fractionation (FFF) is a family of techniques used for the separation of nanoparticles, proteins, DNA, viruses, and other materials based on size, charge, or other physical properties. Separation takes place in a thin, open channel through which samples are transported by a laminar flow of liquid. A field, applied perpendicularly to the channel flow, partitions the various components into regions of different flow velocities.
|
| |
|
Ionization resulting from the effect of a very strong electric field on any particle. The strong electric field may produce ionization in space or in a region very close to a metal or other surface.
Source: IUPAC
|
| |
|
In the flight path of an ion from the source of the mass spectrometer through the mass analyzer to the detector, the ion may pass through regions in which there is no specific magnetic or electric field. These are field-free regions, as in the portion of the ion path between the electric and magnetic analyzers in a double-focusing mass spectrometer, or between the source and the first sector of both single- and double-focusing mass spectrometers. Unimolecular ion dissociations in these regions can occur to give rise to signals in the mass spectrum recorded under normal conditions (metastable ions), or can be specifically investigated.
|
| |
|
|
A parameter that describes the quality of performance of an instrument or an analytical procedure.
|
| |
|
The part of a mixture that passes through a filter, also called permeate.
|
| |
|
Separation of solid particles from a fluid by passing the mixture through a porous, fibrous, or granular substance.
|
| |
|
|
Particulate matter with diameter less than 2.5 microns; PM2.5
|
| |
|
1. Chemical reaction where the rate is directly proportional to the concentration of reactant. 2. Any reaction changing at a constant fractional rate.
|
| |
|
|
The degree to which data produced by a measurement process enables a user to make technically and administratively correct decisions for a stated purpose.
|
| |
|
|
In FID, a detector ionizes hydrocarboncontaining solutes in a hydrogen–air flame. FID is a nearly universal detection technique that responds strongly to most classes of organic compounds.
|
| |
|
|
In FPD, the detector burns heteroatomic solutes in a hydrogen–air flame. The visible-range atomic emission spectrum is filtered through an interference filter and detected with a photomultiplier tube. Different interference filters can be selected for sulfur, tin, or phosphorus emission lines. The flame photometric detector is sensitive and selective.
|
| |
|
|
A device detecting the emission of analyte combustion products generated from a hydrogen-rich flame.
|
| |
|
|
A generic name given to the housing that contains a series of optical components that focus ions onto the detector of a time-of-flight (TOF) mass analyzer. There are basically two different kinds of flight tubes that are used in commercial TOF mass analyzers. One is the orthogonal design, where the flight tube is positioned at right angles to the sampled ion beam, and the other, the axial design, where the flight tube is in the same axis as the ion beam. In both designs, all ions are sampled through the interface region, but instead of being focused into the mass filter in the conventional sequential way, packets (groups) of ions are electrostatically injected into the flight tube at exactly the same time.
|
| |
|
|
Flotation is the removal of matter by entrainment at an interface. In particular, froth flotation is the removal of particulate matter by foaming (frothing).
|
| |
|
Flow Blurring is and efficient technology for pneumatic atomization. Conventional pneumatic nebulizers rely on the venturi effect to produce an aerosol as the nebulizer gas flow is forced through the tip past the narrow bore inner sample capillary. The narrow sample capillary makes it prone to blockages – especially with samples containing high levels of dissolved solids.
Flow Blurring nebulization employs highly turbulent mixing between the nebulizer gas flow and the liquid sample. With no pressure drop and a constant diameter capillary, nebulizer blockage is virtually eliminated. This technique creates an aerosol with extremely fine micro- and nano-scale droplets. It is also compatible with virtually any liquid, and can handle a wide range of solution flow rates.
The Flow Blurring nozzle configuration promotes highly turbulent mixing between the liquid sample and the nebulizer gas flow – creating a fine aerosol of extremely small droplets.
Source: A.M. Ganán-Calvo, Appl. Phys. Lett., 86 (2005) 214101. DOI: 10.1063/1.1931057
|
| |
|
|
Flow injection analysis involves the rapid injection of a sample into a continuously flowing unsegmented carrier stream. If necesaary for detection, one or more reagent solutions are continuously merged with the carrier prior to detection. The injected sample zone undergoes dispersion and is mixed with the carrier and reagent solutions. The resultant product is transported to the detector for measurement.
The dispersion or dilution of the sample zone can be controlled and adapted to the requirements of the analysis, by varying some operational parameters including the injected sample volume, the flow rate of carrier and reagent streams, the reaction coil length, and the inner diameter of the tubing.
|
| |
|
|
Flow injection mass spectrometry is a variant of flow injection
analysis in which a plug of sample is injected into a liquid carrier
stream for on-line analysis by mass spectrometry. This term is synonymous with flow injection analysis mass spectrometry.
|
| |
|
|
A procedure in which the rate of flow of the mobile phase is changed systematically during a part or the whole of the separation.
|
| |
|
Fluorescence is the spontaneous emission of light shortly after the excitation of a material by an electron transition. The fluorescence photons are usually lower in energy than the excitation photons (= Stokes shift). The fluorescence typically has a lifetime (or duration) of nanoseconds (10−9 s) per transition. A fluorescence spectrophotometer normally has an excitation monochromator that defines the excitation energy, and an emission monochromator that provides a full spectrum of the fluorescence emission. Note that fluorescence is the most common interference in Raman spectra and that fluorescence is a strong background interferent of Raman scattering. Often the much stronger fluorescence masks the weaker Raman signal. This is often true for resonance Raman and also for heterogenous biological or mineralogical samples. Also traces of impurities can lead to fluorescence masking the Raman signal.
|
| |
|
Small solid ash particles from the noncombustible portion of fuel that are small enough to escape with the exhaust gases.
|
| |
|
|
Foam fractionation is a method of separation in which a component of the bulk liquid is preferentially adsorbed at the L/V interface and is removed by foaming.
|
| |
|
Detector for spatially disperse ion beams in which all ions simultaneously impinge on the
detector plane.
|
| |
|
Sequence of organisms in an ecosystem occupying specific hierarchical levels (trophic levels) such that organisms belonging to a superior level survive by eating organisms belonging to inferior levels. The chain begins with plants and ends with the largest
carnivores. The sequence can be represented as compartments in a mathematical model or analysis.
Source: IAEA (2000)
|
| |
|
The network of interconnected food chains in an ecosystem.
Source: IAEA (2000)
|
| |
|
|
A forward library search is the process of comparing the mass spectrum
of an unknown compound with spectra in a mass spectral library,
considering only the m/z peaks that have intensities greater than a specific threshold in the unknow.
|
| |
|
|
an accumulation of deposit
|
| |
|
|
process of classification of an analyte or a group of analytes from a certain sample according to physical (e.g., size, solubility) or chemical (e.g., bonding, reactivity) properties
|
| |
|
A fragment ion is the charged product of an ion dissociation. A fragment ion may be stable itself or may dissociate further to form other charged fragment ions and neutral species of successively lower mass. Molecular ions formed in the initial ionization process dissociate to fragment ions because of the excess internal energy that remains after ionization. Note that ions dissociate rather than decompose.
  
|
| |
|
|
When electron transitions (excitation, de-excitation) occur due to electron beams or photons in a molecular system, the time required for the transition is extremely short compared to vibration between atoms. Therefore, the distance between atoms may be considered constant. While the general rotational cycle of molecules is 10-11~ 10-12 sec and the vibrational cycle is 10-13 ~ 10-14 sec, electron tran-sitions are on the order of 10-16 sec. These electron transitions are also called perpendicular transitions.
|
| |
|
|
a synthetic freshwater culture medium specifically designed for studying metal-algae interactions
|
| |
|
A model meant to describe metal-organism interactions determining the uptake, nutrition and toxicity of all cationic trace metals. FIAM assumes that the free metal ion activity reflects the chemical reactivity of the metal, i.e., the extent to what the metal reacts with binding sites on the cell membrane surface, and hence its bioavailability and toxicity
|
| |
|
A procedure in which the sample (liquid or gas) is fed continuously into the chromatographic bed. In frontal chromatography no additional mobile phase is used. Thus, the sample feed is continuously applied to the column and the sample components will displace each other in order of decreasing affinity for the chromatographic medium (this is called sample self-displacement). The least retained solute will be obtained in a pure form (i.e. depleted of the more strongly retained components) until the other solutes break through.
|
| |
|
|
Fourier transform infrared spectroscopy (FTIR) is a technique which is used to obtain an infrared spectrum of absorption, emission, photoconductivity or Raman scattering of a solid, liquid or gas. An FTIR spectrometer simultaneously collects spectral data in a wide spectral range. This confers a significant advantage over a dispersive spectrometer which measures intensity over a narrow range of wavelengths at a time. FTIR technique has made dispersive infrared spectrometers all but obsolete (except sometimes in the near infrared) and opened up new applications of infrared spectroscopy.
|
| |
|
The width (Δm) of a singly charged mass spectral peak (M) at a position corresponding to half of the peak height. It is used to determine the resolving power of a mass spectrometer by the ratio m1/Δm.
|
| |
|
|
Fullerene also called Buckyball or C60 is one of three known pure forms of carbon (graphite and diamond being the other two) that takes a spherical shape with a hollow interior. Buckyballs, named because they resemble the geodesic domes built by architect Buckminster Fuller, were discovered in 1985 among the byproducts of laser vaporization of graphite in which the carbon atoms are arranged in sheets. Though C60, referring to the number of carbon atoms that make up one sphere, is the most common fullerene, researchers have found stable, spherical carbon structures containing 70 atoms (C70), 120 (C120), 180 (C180), and others.
Robert F. Curl Jr. and Richard E. Smalley, both of Rice University in Houston, Texas and Harold W. Kroto of the University of Sussex in England, won the 1996 Nobel Prize for Chemistry for their discovery of buckminsterfullerene, the scientific name for buckyballs.
|
| |
|
|
one of two classes of natural acidic organic polymer that can be extracted from humus found in soil, sediment, or aquatic environments. Its name derives from Latin fulvus, indicating its yellow colour. This organic matter is soluble in strong acid (pH = 1) and has the average chemical formula C135H182O95N5S2.
|
| |
|
|
Functional foods. Foods that affect beneficially one or more target functions in the body, beyond adequate nutritional effects, in a way that is relevant to either an improved state of health and well-being and/or reduction of risk of disease, and they are not pills or capsules, but part of a normal food pattern.
|
| |
|
A group of atoms with characteristic chemical properties that is part of an organic compound, such as nitro, hydroxy or carboxylic acid groups.
|
| |
|