|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
|
A gadolinium-based paramagnetic contrast agent. When placed in a
magnetic field, gadobenate dimeglumine produces a large magnetic moment
and so a large local magnetic field, which can enhance the relaxation
rate of nearby protons; as a result, the signal intensity of tissue
images observed with magnetic resonance imaging (MRI) may be enhanced.
Because this agent is preferentially taken up by normal functioning
hepatocytes, normal hepatic tissue is enhanced with MRI while tumor
tissue is unenhanced. In addition, because gadobenate dimeglumine is
excreted in the bile, it may be used to visualize the biliary system
using MRI.
|
| |
|
|
A gadolinium-based, hydrophilic, macrocyclic, electrically
neutral contrast agent used in contrast-enhanced MRI (CE-MRI).
Gadobutrol is a non-ionic, paramagnetic complex consisting of
gadolinium (Gd3+) chelated with the macrocyclic compound
dihydroxy-hydroxymethylpropyl-tetraazacyclododecane-triacetic acid
(butrol). Following intravenous administration, gadobutrol may increase
MRI sensitivity for the detection of tumors and inflammatory and
demyelinating diseases of the central nervous system (CNS) which are
associated with areas with blood-brain
barrier defects due to altered perfusion or an enlarged extracellular
space. This agent is eliminated in an unchanged form via the kidneys;
extra-renal elimination is negligible.
|
| |
|
Gadolinium is a chemical element that has the symbol Gd and atomic number 64.
Gadolinium is a silvery-white, malleable and ductile rare-earth metal with a metallic lustre. It crystallizes in hexagonal, close-packed alpha form at room temperature, but, when heated to 1508 K or more, it transforms into its beta form, which has a body-centered cubic structure.
Unlike other rare earth elements, gadolinium is relatively stable in dry air. However, it tarnishes quickly in moist air, forming a loosely-adhering oxide which spalls off, exposing more surface to oxidation. Gadolinium reacts slowly with water, and is soluble in dilute acids.
Gadolinium-157 has the highest thermal neutron capture cross-section of any known nuclide with the exception of xenon-135, 49,000 barns, but it also has a fast burn-out rate, limiting its usefulness as a nuclear control rod material.[2]
Gadolinium is strongly paramagnetic at room temperature, and exhibits ferromagnetic properties below room temperature.
Gadolinium demonstrates a magnetocaloric effect whereby its temperature increases when it enters a magnetic field and decreases when it leaves the magnetic field. The effect is considerably stronger for the gadolinium alloy Gd5(Si2Ge2) .
Source: Wikipedia
|
| |
|
|
A coordination complex consisting of a gadolinium ion bound to a
hexadentate organic chelating agent such as
diethylenetriaminepentaacetic acid. Chelates of gadolinium are
frequently utilized as magnetic resonance imaging (MRI) contrast agents
and can be used to track nanoparticle-mediated drug delivery.
|
| |
|
|
A gadolinium chelate paramagnetic contrast agent. When placed in a
magnetic field, gadoterate meglumine produces a large magnetic moment
and so a large local magnetic field, which can enhance the relaxation
rate of nearby protons; as a result, the signal intensity of tissue
images observed with magnetic resonance imaging (MRI) may be enhanced.
Because this agent is preferentially taken up by normal functioning
hepatocytes, normal hepatic tissue is enhanced with MRI while tumor
tissue is unenhanced. In addition, because gadobenate dimeglumine is
excreted in the bile, it may be used to visualize the biliary system
using MRI.
|
| |
|
|
A paramagnetic contrast agent consisting of the disodium salt of the
gadolinium ion chelated with the lipophilic moiety ethoxybenzyl (EOB)
bound to diethylenetriamine pentaacetic acid (DTPA). When placed in a
magnetic field, gadolinium produces a large magnetic moment and so a
large local magnetic field, which can enhance the relaxation rate of
nearby protons; as a result, the signal intensity of tissue images
observed with magnetic resonance imaging (MRI) may be enhanced. Because
this agent is preferentially taken up by normal functioning
hepatocytes, normal hepatic tissue is enhanced with MRI while tumor
tissue is unenhanced. In addition, because this agent is excreted in
the bile, it may be used to visualize the biliary system using MRI.
|
| |
|
|
Electrical signals, measured in volts, can be amplified by appropriate circuitry. The degree of amplification is often called the gain. Thus, an electron multiplier detector can be said to have a gain of 106, indicating that it will amplify the incoming signal one million times.
|
| |
|
Detector effect, occuring at high count rates, that cannot be
mathematically corrected. For high signals, the current flowing over
the dynodes of the electron multiplier is limited, leading to loss in
gain. It is said continuous dynode detector suffers more severe gain
depression effects than discrete detector does, since the continuous
dynode is normally made from a thin film, that does not support high
currents across its total length while discrete dynodes alow for
feeding in the current at each stage.
|
| |
|
Separation of mixtures of compounds in their gaseous state can be achieved by passing them in a stream of heated inert gas, called the carrier gas (usually helium or hydrogen) through a long column containing an immobilised stationary phase for which different components will have different affinities. The column is held in an oven at a carefully controlled temperature. The stationary phase can be chemically bonded to the internal wall of a silica capillary column or can be adsorbed onto a porous inert medium which is packed into a glass spiral column. The sample, either in gaseous form or dissolved in a low boiling solvent, is injected on to the top of the column into a heated chamber and the gaseous mixture is passed through the column. It is common to use a temperature gradient to heat the column. Separated components elute from the end of the column and are passed to a detector.
|
| |
|
GC-MS is a hyphenated technique in which a gas-chromatograph is coupled to a mass spectrometer.
GC-MS can be combined with any ionization method suitable to ionize
neutral molecules in the gas phase but in practice electron
ionization (EI) and chemical ionization (CI) in both positive and
negative ion modes are the methods of choice.
|
| |
|
|
When molecules or ions in the gas phase react and bond with H+ ions, the negative value of the free energy change of the reaction (-ΔG) is expressed as a thermochemical quantity. The protonation re-actions of molecules and ions have different values than proton affinity due to inclusion of chemical structure effects (entropy effect). This has an important role in the generation of multiply charged pro-tonated ions [M+nH]n+ in ESI.
|
| |
|
|
In this technique, solutes partition between a gaseous mobile phase and a liquid stationary phase. Selective interactions between the solutes and the liquid phase cause different retention times in the column.
|
| |
|
|
In this technique, solutes partition between a gaseous mobile phase and a solid stationary phase. Selective interactions between the solutes and the solid phase cause different retention times in the column.
|
| |
|
|
Refers to a symmetrical bell-shaped distribution whose shape is given
by a specific equation (called the normal equation) in which the mean
and standard deviation are variables. It is commonly assumed that the
random error of an analytical method fits the Gaussian distribution and
therefore can be characterized by calculating the standard deviation.
The standard deviation is not a valid statistic if a distribution is not
Gaussian.
|
| |
|
GC-ICP-MS is a hyphenated technique in which a gas-chromatograph is
coupled to the sample inlet of an inductively coupled plasma (ICP) used
as the ionization source for mass spectrometry. Depending on the type
of the mass spectrometer, one or many elements can be monitored in the
GC effluent, obtaining element selective information within the
chromatograms.
|
| |
|
|
(GFC) - size-exclusion chromatography carried out with aqueous mobile phases. Generally refers to separations carried out on soft gels such as polydextrans. Most gel filtration separations involve biopolymers.
|
| |
|
|
(GPC) - size-exclusion chromatography (SEC) carried out with organic mobile phases. Used for the separation and characterisation of polymers. SEC with aqueous mobile phases is referred to as a aqueous GPC, or GFC.
|
| |
|
gaseous elemental mercury - is the less reactive metallic Hg° component of mercury in the atmosphere
|
| |
|
|
The process by which the information in a gene is used to create proteins or polypeptides.
|
| |
|
|
The set of genes of a given organism.
|
| |
|
|
Study of the genome of an organism.
|
| |
|
|
Capable of causing a heritable change to the structure of DNA thereby producing a mutation.
|
| |
|
|
Property of certain toxic substances to produce mutations affecting the genetic makeup of exposed organisms.
|
| |
|
|
The particular genetic pattern seen in the DNA of an individual. ‘Genotype’ is usually used to refer to the particular pair of alleles that an individual possesses at a certain location in the genome. Compare this with phenotype.
|
| |
|
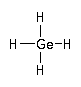 In semiconductor manufacturing germane (GeH 4) is used for the deposition of epitaxial and amorphous silicon-germanium alloy layers (MOCVD).
CAS Number : 7782-65-2
Other names: Germanium hydride; Germanium tetrahydride; Monogermane
|
| |
|
Peaks produced by compounds not present in the original sample. Ghost peaks can be caused
by septum bleed, solute decomposition, or carrier-gas contamination.
|
| |
|
Low-pressure (typically millitorr) discharge that expands to cover essentially the entire surface of an electrode rather than condensing into a narrow arc channel. The glow discharge source (GDS) is used as a source both for atomic emission specrometry (GDOES) or mass spectrometry (GDMS).
|
| |
|
|
A detoxifying selenoenzyme responsible for preventing damage caused by oxidative stress. Its active site is the Se atom on a Se-Cysteine residue, and it functions by reducing hydroperoxides and organic peroxides.
|
| |
|
|
A single sample collected at a
particular time and place that represents the composition of the probed entity only at that time
and place.
|
| |
|
|
An HPLC procedure where the composition of the mobile phase changes during the separation; opposite of isocratic elution where the mobile phase composition remains constant throughout the entire chromatogram. A typical example would be where the mobile phase starts out as water, and methanol is added continuously so that the percentage of methanol in the mobile phase increases from zero to 100% during the chromatographic run.
|
| |
|
The graphite furnace is used as an electrothermal atomizer for atomic absorption spectrometry ( ETAAS) or as a vaporizer for sample introduction to plasma sources for atomic spectrometry such as ICP-OES, ICP-MS.
In graphite furnace analysis, a measured amount of a sample (liquid, sluirry or solid) is placed inside the furnace cup or tube and subjected to a multi-step temperature programme including drying and ashing steps. After such thermal pre-treatment the sample is vaporized or even atomized either for direct detection in the gas phase or transport to the plasma source.
|
| |
|
|
A spectrometer that uses a grating for the diffraction of light and the resulting dispersion of light into various wavelengths. This is often termed a monochromator, meaning an instrument able to resolve “one color,” one wavelength, or a single limited set of wavelengths.
|
| |
|
|
conversion a chemical compound into a derivative (addition of e.g. alkyl group) using a Grignard reagent
|
| |
|
|
A Grignard Reagent is an alkyl- or aryl- magnesium halide. This reagent is important in the synthesis of carbon-carbon bonds in the Grignard reaction. Victor Grignard, of the University of Lyons, won the 1912 Nobel Prize in Chemistry for his discovery of Grignard reagents. Grignard reagents are formed by reacting alkyl or aryl halides with organomagnesium metal, conferring a negative charge on the terminal carbon, a rare occurrence. Bromides are most often used, as they work the fastest and are readily available among halides, iodide and chloride are also used, while fluoride is generally unreactive towards organomagnesium compounds. The Grignard reaction is exothermic and because of a oxide layer present on the magnesium, the start of the reaction is sometimes delayed. A crystal of iodine is often introduced to initiate the reaction.
|
| |
|
|
Water beneath the earth's surface in the spaces between soil particles and between rock surfaces [compare with surface water].
|
| |
|
|
A short column placed between the sample injector and the inlet of the main ("analytical") column. The guard column is typically packed with the same kind of stationary phase as the main column, and is intended to absorb or pick up impurities (chemical garbage) in the sample or mobile phase that might damage the main column. It is also sometimes called a pre-column.
|
| |
|