|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
The time it takes for half the original amount of a substance to disappear.
In the environment, the half-life is the time it takes for half the original
amount of a substance to disappear when it is changed to another chemical
by bacteria, fungi, sunlight, or other chemical processes. In the human
body, the half-life is the time it takes for half the original amount
of the substance to disappear, either by being changed to another substance
or by leaving the body. In the case of radioactive material, the half
life is the amount of time necessary for one half the initial number of
radioactive atoms to change or transform into another atom (that is normally
not radioactive). After two half lives, 25% of the original number of
radioactive atoms remain.
Source: ATSDR Glossary of Terms
|
| |
|
|
Formation of gas-phase ions accompanied by extensive fragmentation
|
| |
|
|
Set of inherent properties of a substance, mixture of substances, or a process involving substances that, under production, usage, or disposal conditions, make it capable of causing adverse effects to organisms or the environment, depending on the degree of exposure; in other words, it is a source of danger.
|
| |
|
|
Hexabromocyclododecane (HBCD or HBCDD) is a brominated flame retardant.
Its primary application is in extruded (XPS) and expanded (EPS)
polystyrene foam that is used as thermal insulation in the building
industry. HBCD is highly efficient in this application so that very low
levels are required to reach the desired flame retardancy. Typical HBCD
levels in EPS are 0.7% and in XPS 2.5%.
|
| |
|
|
Gas-phase sampling technique in which solute is removed from an enclosed space above a solid or liquid sample.
|
| |
|
|
"Health Canada is the federal department responsible for helping the people of Canada maintain and improve their health ."
"In
partnership with provincial and territorial governments, Health Canada
provides national leadership to develop health policy, enforce health
regulations, promote disease prevention and enhance healthy living for
all Canadians."
See also the Health Canada Environment page:
www.hc-sc.gc.ca/english
(Source: Health Canada website )
|
| |
|
|
Heavy ion induced desorption (HIID) is the general name for particle bombardment ionization methods using beams of high energy atoms or molecules (20 ~ 300Da) accelerated on the MeV level.
|
| |
|
|
A very imprecise term, never defined by any authoritive body, used loosely to refer to both the element and its compounds. It is based on categorization by density, which is rarely a biologically significant property. It is often used as a group name for metals and metalloids that have been associated with contamination and potential toxicity or ecotoxicity. However, the assumption that all so-called "heavy metals" and their compounds have highly toxic or ecotoxic properties is not supported by facts. Even further, the list of "heavy metals" is not clearly defined and has no basis in their chemistry. Even the term "metal" is commonly misused in both toxicological literature and in legislation to mean the pure metal and all the chemical species in which it may exist.
Very often, ther term "heavy metals" is used, based on one of the following "definitions":
- In terms of density: Metals having a density greater than 4 (4.5, 5 or 6 g/cm3)
- In terms of atomic weight: Metals with a atomic weight greater than 23 (40)
- In terms of atomic number: Metals with an atomic number greater than 20
The use of the term "heavy metals" is highly discorouged by IUPAC.
|
| |
|
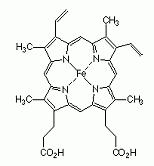 A near-planar coordination complex obtained from iron and the dianionic form of porphyrin. Derivatives are known with substituents at various positions on the ring named a, b, c, d etc. Heme b, derived from protoporphyrin IX, is the most frequently occurring heme. A near-planar coordination complex obtained from iron and the dianionic form of porphyrin. Derivatives are known with substituents at various positions on the ring named a, b, c, d etc. Heme b, derived from protoporphyrin IX, is the most frequently occurring heme.
|
| |
|
|
Hemin is an iron containing metalloporphyrin. Specifically, the Fe3+ oxidation product of heme is termed hemin. Hemin acts as a feed-back inhibitor on ALA synthase. Hemin also inhibits transport of ALA synthase from the cytosol (its' site of synthesis) into the mitochondria (its' site of action) as well as represses synthesis of the enzyme. Hemin is also known as a drug that is derived from processed red blood cells. Hemin for injection was known previously as hematin.
|
| |
|
|
An insoluble iron-protein complex that comprises a storage form of iron mainly in the liver, spleen and bone marrow.
|
| |
|
|
a cyclic or ring molecular structure in which one
or more of the atoms in the ring is an element other than carbon.
(Common heterocyclics are pyridine, pyrrole, furan, thiophene, and
purine).
|
| |
|
|
The degree to which a property or a constituent is randomly distributed throughout a quantity of material. The degree of heterogeneity is the determining factor of sampling error.
|
| |
|
A device containing six rods used as an ion guide to focus divergent beams of ions, as a storage device to accumulate ions before transfer to another part of the system, or as a collision cell. RF voltages are generally applied to the rods. See also octapole
|
| |
|
|
- divalent mercury - the dominating mercury form in organic and inorganic mercury compounds. In the atmosphere, mercury species with divalent mercury are more easily washed out of the air with precipitation and deposited than elemental mercury;
|
| |
|
|
Hg(p) - particulate mercury - mercury bound in, or adsorbed on, particulate material. In the atmosphere, particulate mercury is deposited much faster than elemental mercury;
|
| |
|
|
Also known as magnetic sector, sector field or double focusing ICP-MS. Magnetic sector based ICP-MS instruments are capable of resolution (M/ΔM) of up to 10,000 and are able to resolve most polyatomic species from analytes at the same nominal mass.
|
| |
|
|
Condition of being of uniform structure or composition with respect to one or more specified properties. A reference material is said to be homogeneous with respect to a specified property if the property value, as determined by tests on samples of specified size, is found to lie within the specified uncertainty limits, the samples being taken either from different supply units (bottles, packages, etc.) or from a single supply unit.
|
| |
|
|
one of two classes of natural acidic organic polymer that can be extracted from humus found in soil, sediment, or aquatic environments. The process by which humic acid forms in humus is not well understood, but the consensus is that it accumulates gradually as a residue from the metabolism of microorganisms. Humic acid is the humic fraction soluble in alkali, but not in acid. The other organic polymer is fulvic acid.
|
| |
|
|
operationally defined fraction of natural organic matter
|
| |
|
|
A programme for the computation of chemical equilibrium composition of aqueous batch systems including surface complexation modelling of ion adsorption at the oxide/solution interface.
|
| |
|
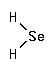 Hydrogen selenide is a metabolite within the metabolic pathway of selenium.
CAS Number : 7783-07-5
Other names: Selenium hydride
|
| |
|
|
Phosphorus in the sample as measured by the sulfuric acid hydrolysis procedure, and minus pre-determined orthophosphates (EPA, 1979). Includes dissolved and particulate condensed phosphate that is converted to dissolved orthophosphate through acidification of the sample (APHA, 1989). It is referred to as Dissolved hydrolyzable P or Total hydrolyzable P, when measured on filtered or unfiltered sample, respectively.
|
| |
|
Having an affinity for water; attracting, dissolving in, or absorbing water; readily absorbing moisture; having strongly polar molecular groups that readily interact with water.
|
| |
|
Insoluble in water; the extent of insolubility; not readily absorbing water; resisting or repelling water, wetting, or hydration; or being adversely affected by water.
|
| |
|
A type of hydrophobic interaction chromatography (HIC) that is based on pH rather than salt concentration, allowing for elution under relatively mild conditions amd eliminating the requirement for an associated filtration step in early separations.
|
| |
|
A type of liquid chromatography tha makes use of the relative solubility of proteins and matrix materials. Hydrophobic interactions are strongest at high ionic strengths, so salt is usually included to increase those levels.
|
| |
|
Water above, on, or in the Earth’s crust, including oceans, seas, lakes, groundwater, and atmospheric
moisture.
|
| |
|
|
Hydroxy-oxide is a chemical compound containing oxygen (O), the hydroxyl (OH)
anion and some other chemical element. For example, NiOOH is the chemical
formula for nickel hydroxy-oxide, and FeOOH that of iron hydroxy-oxide.
|
| |
|
|
The term hyperaccumulator, referring to a plant with a highly abnormal level of metal accumulation, was originally coined to describe a plant with a concentration exceeding 0.1% Ni (dry mass) and then extended to other metals such as Co and Pb (0.1% threshold value) and Zn and Mn (1% threshold value). To date ~400 known metal hyperaccumulators have been reported worldwide. The most widely referred to have been the mustard (Thlaspi caerulescens) containing 1-2% Zn, Alyssum lesbiacum (>1% of Ni), and the New Caledonian tree Sebertia acuminata whose latex was reported to contain above 20% Ni (dry mass).
The discovery of such metal-hyperaccumulating properties in certain plants spurred research towards using them for cleanup of heavy-metal-contaminated soils.
|
| |
|
|
The term "hyphenated techniques", introduced by Hirschfeld, refers to an online combination of a chromatographic (later also electrophoretic) separation technique with a sensitive element or molecule specific detector.
see: T. Hirschfeld, Anal. Chem., 52 (1980) 297 A
NB!: A slash is sometimes used instead of hyphen, especially if the name of one of the methods contains a hyphen itself.
|
| |
|