|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
|
a substance added to both samples and standards in such a concentration that largely exceeds the concentration initially present in the substance in the sample. The reason for addition of a radiation buffer is to make the interference from the initial substance in the sample insignificant since now a large excess is added to both sample and standard.
|
| |
|
|
atoms or molecular entity possessing an unpaired electron
|
| |
|
|
Discharge caused by 23 ~ 30kV with high frequencies of 200 kHz or more
|
| |
|
A radioactive isotope. An unstable isotope of an element that decays or
disintegrates spontaneously, emitting radiation. More that 1300 natural
and artificial radioisotopes have been identified.
|
| |
|
|
A radioactive species of an atom. The term nuclide implies an atom of specified atomic number and mass number. These may be subdivided into natural
radionuclides such as radium or uranium which are normally present in
the earth, or artificial radionuclides which are not normally present
(or normally present in very small amounts) and are produced by nuclear
fission.
In the study of biochemical processes, radioactive isotopes are used for labelling compounds that subsequently are used to investigate various aspects of the reactivity or metabolism of proteins, carbohydrates and lipids or as sources of radiation in imaging. The fate of the radionuclide in reactive products or metabolites is determined by following (counting) the emitted radiation. In analytical chemistry radionuclides are used as tracers for the localization of the analyte or in yield experiments.
|
| |
|
|
Radiopharmaceuticals are drugs that contain, among other ingredients, radioactive forms of chemical elements called radioisotopes. Depending on the type of radiation
that those radioisotopes produce, they can be used to diagnose or treat
several medical conditions. Their applications range from imaging of
many different organs, such as brain, heart, kidney and bone, to the
treatment of cancer and hyperthyroidism.
|
| |
|
|
Random Errors vary in sign and magnitude and are
unpredictable. They occur by chance. Random errors average out and
approach zero if enough measurements are made.
|
| |
|
|
Comprise 17 elements: Sc, Y, La, Ce, Pr, Nd, (Pm), Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu.
|
| |
|
|
Limiting droplet size at which self-fragmentation will occur with static charge droplets generated in ESI or other ionization processes. Self-fragmentation occurs when the Coulombic repulsion force generated by the excess charge in a droplet exceeds the surface tension maintaining the droplet. Vaporization of electrons and ions from the static charge droplet surface occurs even with droplets having large sizes. As the size becomes even smaller, the surface tension begins to squeeze the droplet and promote further vaporization.
|
| |
|
|
REACH is the acronym for the European regulatory framework for the "Registration, Evaluation and Authorisation of Chemicals". The aim of this legislation is to improve the protection of human health and the environment through the better and earlier identification of the properties of chemical substances.
|
| |
|
RGM is defined as water-soluble mercury species with sufficiently high vapor pressure to exist in the gas phase. The reactive term refers to the capability of stannous chloride to reduce these species in aqueous solution without pretreatment. The most likely candidate for RGM species is HgCl2 and possibly other divalent mercury species. RGM is deposited readily in rainfall.
|
| |
|
|
“Easily reducible,” determined by SnCl2 reduction on acidified samples,
includes inorganic complexes, labile organic associations, elemental
mercury, and labile particulate mercury, doesn't include C-Hg bound
mercury such as methylmercury and dimethylmercury. Same as acid labile
mercury.
|
| |
|
Molecules including peroxides, hydroxyl radicals, and superoxide may oxidatively damage proteins. Many selenoproteins are able to decompose or reduce ROS. While the traditional view is that selenoproteins thus protect cellular structures from oxidative damage, a more modern view stresses the signaling roles of ROS and oxidatively modified proteins. Selenoproteins may thus contribute to regulation of cellular
physiology in addition to a “protective” role against oxidative damage.
|
| |
|
Soluble reactive Phosphate (SRP) or Total Reactive Phosphate (TRP)
Phosphorus form that responds to colorimetric test without preliminary hydrolysis or digestion. Although reactive phosphate is comprised largely of orthophosphates, it may include easily hydrolyzable inorganic and organic forms of P (APHA, 1989, Baldwin, 1998). Reactive phosphate maybe measured in both filtered* (dissolved SRP, the most commonly measured form of SRP) or unfiltered (total reactive phosphate) (EPA, 1979).
|
| |
|
|
A solution obtained by carrying out all of the steps of the analytical procedure in the absence of a sample.
|
| |
|
A rearrangement ion is a fragment ion formed in a dissociation in which atoms or groups of atoms have transferred from one part of the molecule to another during the fragmentation process. Because the structural requirements to form rearrangement fragment ions are constrained, the identification and rationalization of rearrangement fragment ions are especially important in spectral interpretation.
  
|
| |
|
|
Restoration to original form of a substance previously altered for preservation and storage.
|
| |
|
A normal mass spectrometric data set consists of full mass spectra recorded sequentially in time as sample is admitted to the ion source, either from a direct-insertion probe or from a chromatograph (for example, GC/MS). The total ion current trace is the sum of ion abundances in each mass spectrum plotted versus time. Clearly each mass spectrum will also contain a pattern of molecular and fragment ions. Ions of these particular masses can be specified, and the data system can plot the scan-by-scan abundances of these specific ions versus time, which is known as a reconstructed ion chromatogram. The reconstructed ion chromatogram can be used to identify all ions that belong together in a single mass spectrum by virtue of their coincident peaks in time (and discriminate against background ions), and can also be used to screen a GC/MS run for related classes of compounds by reconstruction of ion chromatograms for common structurally specific ions.
  
|
| |
|
|
The fraction of the total quantity of a substance recoverable following a chemical procedure.
|
| |
|
|
A method validation experiment
performed to estimate proportional systematic error. A test sample is
prepared by adding a standard solution of the analyte of interest to an
aliquot of a patient specimen. A baseline sample is prepared by adding
an equal amount of diluent or solvent to the same patient specimen. The
two samples are analyzed and recovery estimated from the difference
observed between the two samples divided by the amount added.
|
| |
|
|
Any oxidation-reduction (redox) reaction can be divided into two half reactions: one in which a chemical species undergoes oxidation and one in which another chemical species undergoes reduction. If a half-reaction is written as a reduction, the driving force is the reduction potential. If the half-reaction is written as oxidation, the driving force is the oxidation potential related to the reduction potential by a sign change. So the redox potential is the reduction/oxidation potential of a compound measured under standard conditions against a standard reference half-cell. In biological systems the standard redox potential is defined at pH = 7.0 versus the hydrogen electrode and partial pressure of hydrogen = 1 bar. See also electrode potential.
|
| |
|
|
Also called a reducing agent. A substance that causes another substance to be reduced by donating one or more electrons to it (compare with oxidant).
|
| |
|
|
An enzyme that catalyzes the reduction of a molecule.
|
| |
|
|
The addition of hydrogen, removal of oxygen, or addition of electrons to an element or compound.
|
| |
|
|
A method of separating certain elements by precipitation, using a reducing agent in the chemical reaction.
|
| |
|
|
Material or substance with one or more property values that are sufficiently homogeneous and well established to be used for calibration of an apparatus, assessment of a measurement method, or assigning values to materials.
|
| |
|
|
Thoroughly investigated method, clearly and exactly describing the necessary conditions and procedures, for the measurement of one or more property values that has been shown to have accuracy and precision commensurate with its intended use and that can therefore be used to assess the accuracy of other methods for the same measurement, particularly in permitting the characterization of an reference material.
|
| |
|
A reflectron is a device incorporated into the flight tube of a time-of-flight mass spectrometer that operates as an electrostatic mirror. Ions with a range of kinetic energies from the ionization source traverse the first portion of the flight tube and then spend different amounts of time in the reflectron. The net result is that these (reflected) ions all come into focus at the detector located at the end of the second portion of the flight tube, leading to higher mass resolution.
  
|
| |
|
Change of direction of light as it passes between dissimilar materials.
|
| |
|
The relative abundance of an ion is the measured intensity for the ion beam at that designated m/z value. To be precise, ion beams have intensities, and ions have abundances. Relative abundance is a term related to the practice of assigning the most abundant ion in a measured and plotted mass spectrum a relative abundance of 100% and normalizing all other ion abundances to that value.
  
|
| |
|
|
In mass spectrometry relative intensity designates the ratio between the intensity of the ion beam for a certain mass and the maximum intensity of the ion beam. Normally this ratio is expressed using the heights of each peak on a spectrum with the highest peak defined as 100. Another method defines the total ion quantity or the ion quantity in a specific region as 100. The peak height method is also called the pattern coefficient while the ion quantity method is called the %? method. With the ion peak intensity terms for ion quantity (abundance), ion peak strength (intensity), peak height (height) and peak surface area (area) are also used.
|
| |
|
Repeatability is the precision obtained under repeatability conditions.
Repeatability is the closeness of the agreement between the results of independent measurements of the same analyte carried out subject to all of the following conditions: the same method of measurement, the same observer, the same measuring instrument, the same location, the same conditions of use, repetition over a short period of time. Independent measurements are made on distinct subsamples of a test material. If possible, at least 8 measurements should be performed. Repeatability is a characteristic of a method not of a result.
|
| |
|
|
The repeller voltage is the voltage applied to an electrode to push generated ions from the ion chamber toward the analyzer of a mass spectrometer.
|
| |
|
Reproducibility is the precision under reproducibility conditions.
Reproducibility is the closeness of the agreement between the results of measurements of the same analyte in distinct subsamples of a test material, where the individual measurements are carried out changing conditions such as: observer, measuring instrument, location, conditions of use, time, but applying the same method.
  
|
| |
|
Separation of signals that should, under some circumstance, be distinguishable. Relative resolution (unitless) is the value of the distinguishable parameter (say, wavelength) divided by the smallest value difference that an experiment can actually distinguish. Absolute resolution (has units) is the smallest difference that can be discerned in a measured quantity.
|
| |
|
|
The resolution of a mass spectrometer is defined in several different ways relative to the commonly given formula of
m/Δm, where m is the mass of the ion at which resolution is specified. For two
adjacent, symmetric peaks of equal height in a mass spectrum, the instrumental
(physical or electrical) parameters are adjusted such that the peaks at masses
m and (m -Δm) are separated by a valley that, at its lowest point, is just 10%
of the height of either peak. Then, the resolution (10% valley definition) is m/Δm.
The definition can be given also for 50% valley or 5% valley separations. For a
single peak, the resolution is still calculated as m/Δm, but now Δm is the
width of the peak at a height that is a specified fraction of the maximum peak height.
A 5% peak width definition is technically equivalent to the 10% valley
definition of resolution. A common standard is the definition of resolution
based on Δm being the full width of the peak at half its maximum height (FWHM).
|
| |
|
|
Mass fraction of dust (particles) that penetrates to the unciliated airways of the lung (the alveolar region): it is represented by a cummulative log-normal curve having a median aerodynamic diameter of 4.25 µm and a standard deviation of 1.5 (values for humans).
|
| |
|
|
Retention time ( tR), also called the total retention time. The time between injection and the appearance of the peak maximum. The total retention volume (VR) is determined by multiplying the retention time by the flow rate. The adjusted retention time (tR') adjusts for the column void volume; tR' = tR - tM. It usually is measured from the point of injection to the apex of the peak, but it should be measured to the center of gravity of the peak for asymmetric peaks..
|
| |
|
|
The most common form of HPLC, in which water-based/polar mobile phases are used with special column packings that have a hydrophobic surface layer.
|
| |
|
|
use of plants to accumulate compounds from aqueous solutions in roots.
|
| |
|
|
Identification and quantification of the risk resulting from a specific use or occurence of an agent, taking into account possible harmful effects on individuals exposed to the agent in the amount and manner proposed and all the possible routes of exposure.
Note: Quantification ideally requires the establishement of dose-effect and dose-response relationships in likely target individuals and populations.
|
| |
|
A measure of the capacity of an analytical procedure to remain unaffected by small, but deliberate variations in method parameters, and which provides an indication of its reliability during normal usage.
|
| |
|
|
Removal of solvents by distillation under vacuum.
|
| |
|
Organoarsenical (3-nitro-4-hydroxyphenylarsonic acis, 3-NHPAA)
used as s feed additive for broiler chickens in order to
control coccidial intestinal parasites, thereby improving feeding
efficiency.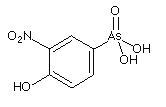
|
| |
|
|
Ruggedness is the degree of concordance between test results obtained
for the same samples under various conditions – for example, in
different laboratories or using different instruments.
|
| |
|
|
the portion of rainfall, melted snow, or irrigation water that flows
across ground surface and eventually is returned to streams. Runoff can
pick up pollutants from the air or the land and carry them to the
receiving water.
|
| |
|