A group of Chinese researchers propose a new system for coupling µHPLC to ICP-MS. The new system avoids any post-column dispersion, maintaining the molecular resolution obtained on μHPLC and the limit of detection (LOD) of ICP-MS.
Background:
Inductively coupled plasma mass spectrometry (ICP-MS) is a very powerful elemental detection system, that allows for sensitive determination of most elements. The hot environment of the inductively coupled plasma (ICP) enables the atomization of molecules and the ionization of atoms supporting compound independent calibration. Unfortunately, it also destroys all molecular information. In case that information about the element containing compounds is required, the ICP-MS has to be coupled with a separation system. The most versatile separation system for such speciation analysis is high-performance liquid chromatography (HPLC). Since HPLC works with liquid samples and the standard sample introduction system of the ICP is for liquid samples, coupling of the two systems seems to be relatively straight forward. Conventional interfacing is achieved by directing the effluent of the HPLC column into the nebulizer of the ICP-MS sample introduction system. In order to optimize the coupling, the nebulizer with a working flow-rate equal to the effluent flow rate should be selected. Even then one issue remaining is that the separated analyte-containing chromatographic peaks broaden and even remix prior to mass spectrometric quantification due to the inevitable molecular diffusion within the dead-volume introduced by the coupling components, especially the transfer capillary and the spraychamber.
The new study:
A group of Chinese researchers recently presented a new system for coupling µHPLC to ICP-MS, which they named "zero-interfacing". The system is based on a self-designed direct injection nebulizer with a tapered nozzle, in which a capillary chromatographic column can be harboured as the sample capillary while an Ar gas flow is blown through the nozzle mouth.
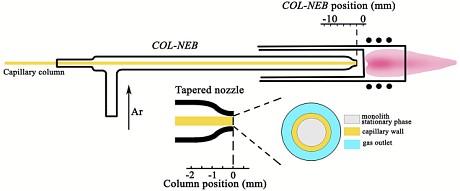
Figure: Schematic diagram of the COL-NEB for zero-interfacing µHPLC to ICP-MS
The column nebulizer assembly (COL-NEB) can be positioned just before the base of the Ar-plasma, with its gas flow creating the central sampling channel of the ICP torch for online nebulization and transportation of the analytes separated on μHPLC into the plasma. By avoiding any dead-volume for the hyphenation and the dispersion coming with the dead-volume, the full width at half-maximum of a SLUGT peptide chromatographic peak was reduced to 1.71 ± 0.07 s (n = 5) with a 0.72 fg LOD (3σ) of 80Se. The authors also reported the determination of 32 Se-containing peptides in the trypsin lysate of the water-soluble fraction (≥3000 MW) from Se-enriched yeast
CRM SELM-1 within a 10-min run, the highest record to date.
The authors conclude with their belief that their approach paves the way to determining accurate information on a heteroatom and its binding biomolecules that play key roles during life processes.
Comment:
For those who are not familiar with direct injection nebulizers, we would like to emphasize that the distance of the nebulizer nozzle from the plasma base is critical. A too small distance from the hot plasma will melt the nebulizer nozzle, thereby destroying the nebulizer. Special attention is necessary during the ignition phase of the plasma.
 The original study
The original study

Yang Zhou, Xingrui Song, Xiaowen Yan, Limin Yang, Shi Chen, and Qiuquan Wang,
Zero-Interfacing μHPLC to ICPMS, Anal. Chem. 94/49 (2022) 16975–16979.
DOI: 10.1021/acs.analchem.2c03951 Related studies (newest first)
Related studies (newest first)
 M. Bernardin, F. Bessueille-Barbier, A. Le Masle, C.-P. Lienemann, S.
Heinisch, Suitable interface for coupling liquid chromatography to
inductively coupled plasma-mass spectrometry for the analysis of organic
matrices. 2. Comparison of Sample Introduction Systems, J. Chromatogr. A, 1603 (2019) 68-80. DOI:
10.1016/j.chroma.2019.04.074
M. Bernardin, F. Bessueille-Barbier, A. Le Masle, C.-P. Lienemann, S.
Heinisch, Suitable interface for coupling liquid chromatography to
inductively coupled plasma-mass spectrometry for the analysis of organic
matrices. 2. Comparison of Sample Introduction Systems, J. Chromatogr. A, 1603 (2019) 68-80. DOI:
10.1016/j.chroma.2019.04.074
 M. Bernardin, F. Bessueille-Barbier, A. Le Masle, C.-P. Lienemann, S. Heinisch, Suitable
interface for coupling liquid chromatography to inductively coupled
plasma-mass spectrometry for the analysis of organic matrices. 1.
Theoretical and experimental considerations on solute dispersion, J. Chromatogr. A, 1565 (2018) 68-80. DOI: 10.1016/j.chroma.2018.06.024
M. Bernardin, F. Bessueille-Barbier, A. Le Masle, C.-P. Lienemann, S. Heinisch, Suitable
interface for coupling liquid chromatography to inductively coupled
plasma-mass spectrometry for the analysis of organic matrices. 1.
Theoretical and experimental considerations on solute dispersion, J. Chromatogr. A, 1565 (2018) 68-80. DOI: 10.1016/j.chroma.2018.06.024
 R. Milacic,
R. Milacic, T. Zuliani, J. Vidmar,
J. Scancar, Monolithic chromatography in speciation analysis of metal-containing biomolecules: a review, J. Anal. At. Spectrom., 31/9 (2016) 1766-1779.
DOI: 10.1039/c6ja00121a 
Christina Rappel,
Dirk Schaumlöffel,
Improved nanonebulizer design for the coupling of nanoHPLC with ICP-MS, J. Anal. At. Spectrom., 25 (2010) 1963–1968.
DOI: 10.1039/c0ja00050g
 Kirk E. Lokits, Patrick A. Limbach, Joseph A. Caruso, Interfaces for capillary LC with ICPMS detection: A comparison of nebulizers/spray chamber configurations, J. Anal. At. Spectrom., 24/4 (2009) 528-534. DOI: 10.1039/b820121h
Kirk E. Lokits, Patrick A. Limbach, Joseph A. Caruso, Interfaces for capillary LC with ICPMS detection: A comparison of nebulizers/spray chamber configurations, J. Anal. At. Spectrom., 24/4 (2009) 528-534. DOI: 10.1039/b820121h
 P. Giusti
P. Giusti,
R. Lobinski,
J. Szpunar, D. Schaumlöffel, Development of a Nebulizer for a Sheathless Interfacing of NanoHPLC and ICPMS. Anal. Chem., 78/3 (2006) 965– 971.
DOI: 10.1021/ac051656j
S. Groom, G. Schaldach, M. Ulmer, P. Walzel, H. Berndt,
Adaptation of a New Pneumatic Nebulizer for Sample Introduction in ICP Spectrometry. J. Anal. At. Spectrom., 20/3 (2005) 169– 175.
DOI: 10.1039/b410772c
J. Mora, S. Maestre, V. Hernandis, J.L. Todolí,
Liquid-Sample Introduction in Plasma Spectrometry. TrAC Trends Anal. Chem., 22 (2003) 123– 132.
DOI: 10.1016/S0165-9936(03)00301-7  D. Schaumlöffel,
D. Schaumlöffel, J.R. Encinar,
R. Lobinski, Development of a Sheathless Interface between Reversed-Phase Capillary HPLC and ICPMS via a Microflow Total Consumption Nebulizer for Selenopeptide Mapping. Anal. Chem., 75/24 (2003) 6837– 6842.
DOI: 10.1021/ac034819h
M. Wind, A. Eisenmenger, W.D. Lehmann,
Modified Direct Injection High Efficiency Nebulizer with Minimized Dead Volume for the Analysis of Biological Samples by Micro- and Nano-LC-ICP-MS. J. Anal. At. Spectrom., 17/1 (2002) 21– 26.
DOI: 10.1039/b108153p 
B.W. Acon, J.A. McLean,
A.A. Montaser, A Direct Injection High Efficiency Nebulizer Interface for Microbore High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom. 2001, 16, 852– 857.
DOI: 10.1039/b103085j 
J. Li, T. Umemura, T. Odake, K. Tsunoda,
A High-Efficiency Cross-Flow Micronebulizer for Inductively Coupled Plasma Mass Spectrometry, Anal. Chem., 73/7 (2001) 1416– 1424,
DOI: 10.1021/ac001282o 
J.A. McLean, H. Zhang,
A.A. Montaser, A Direct Injection High-Efficiency Nebulizer for Inductively Coupled Plasma Mass Spectrometry. Anal. Chem., 70/5 (1998) 1012– 1020.
DOI: 10.1021/ac9708553

Anders Tangen, Roger Trones, Tyge Greibrokk,
Walter Lund,
Microconcentric Nebulizer
for the Coupling Of Micro Liquid Chromatography and Capillary Zone
Electrophoresis With Inductively Coupled Plasma Mass Spectrometry, J. Anal. At. Spectrom., 12/6 (1997) 667-670.
DOI: 10.1039/a607623h  S.A. Pergantis
S.A. Pergantis, E.M. Heithmar, T.A. Hinners,
Microscale Flow Injection and Microbore High-Performance Liquid Chromatography Coupled with Inductively Coupled Plasma Mass Spectrometry via a High-Efficiency Nebulizer. Anal. Chem., 67/24 (1995) 4530– 4535.
DOI: 10.1021/ac00120a016 
S.H. Nam, J.S. Lim,
A. Montaser, High-Efficiency Nebulizer for Argon Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectrom., 9/12 (1994) 1357– 1362.
DOI: 10.1039/ja9940901357 
S.C.K. Shum, H. Pang, R.S. Houk,
Speciation of Mercury and Lead Compounds by Microbore Column Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry with Direct Injection Nebulization. Anal. Chem., 64/20 (1992) 2444– 2450.
DOI: 10.1021/ac00044a025 
Barry L. Sharp,
Pneumatic Nebulisers and Spray Chambers for Inductively Coupled Plasma Spectrometry: a Review. Part 2. Spray Chambers. J. Anal. At. Spectrom. 3/7 (1988) 939– 963.
DOI: 10.1039/ja9880300939 
K.E. Lawrence, G.W. Rice, V.A. Fassel,
Direct Liquid Sample Introduction for Flow Injection Analysis and Liquid Chromatography with Inductively Coupled Argon Plasma Spectrometric Detection. Anal. Chem., 56/2 (1984) 289– 292.
DOI: 10.1021/ac00266a038
 Related EVISA Resources
Related EVISA Resources
 Related EVISA News
Related EVISA News
last time modified: January 11, 2025