|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
ICP-MS (Inductively Coupled Plasma Mass Spectrometry) is a type of mass spectrometry that is highly sensitive and capable of simultaneous determination of a range of elements at below one part in 10 12. It is based on coupling together an inductively coupled plasma as an ion source with a mass spectrometer as a detection system for ions.
The ions generated within the plasma at atmospheric pressure are extracted through a series of cones into the vacuum chamber of a mass spectrometer, usually a quadrupole. Other mass analyzers coupled to ICP systems include double focusing magnetic-electric sector systems with both single and multiple collector, as well as time-of-flight (TOF) systems (both with axial and orthogonal accelerators). In any case the ions are separated on the basis of their mass-to-charge ratio and a detector receives an ion signal proportional to the concentration.
The concentration of a sample can be determined through calibration with elemental standards (external calibration). Accuracy and precision can be enhanced by using internal standards and rationing the measurements against these. ICP-MS also lends itself to quantitative determinations through isotope dilution analysis, a single point method based on an isotopically enriched standard.
|
| |
|
|
"Inductively Coupled Plasma-Optical Emission spectroscopy" (ICP-OES) often also called ICP-AES (Inductively Coupled Plasma-Atomic Emission spectroscopy) is an atomic spectrometric detection technique used for the quantitative determination of elemental concentrations. The technique is using the Inductively coupled plasma (ICP) as an atomizer and excitation source, that due to its very high temperature in the range of 7000-8000K efficiently desolvates, vaporizes, excites, and atomizes samples introduced as gases, vapors or aerosols.
|
| |
|
In a MALDI-TOF instrument, the incident laser desorption and ionising beam can be rastered over a two-dimensional surface. Similarly, in TOF SIMS, the impacting ion beam is rastered. Diagnostic ions specific to target molecules are detected and their abundance is converted into an image reflecting their intensities within the material being analysed. This development has important applications in biological and medical studies and has been used as a complementary technique to microscopy for tissue sections. Also known as ion imaging.
|
| |
|
|
- a device in which particles are removed by impacting the aerosol particles into a liquid.
|
| |
|
|
In an artificial environment outside a living organism or body. For example, some toxicity testing is done on cell cultures or slices of tissue grown in the laboratory, rather than on a living animal [compare with in vivo].
|
| |
|
|
Within a living organism or body. For example, some toxicity testing is done on whole animals, such as rats or mice [compare with in vitro].
|
| |
|
|
Collision induced dissociation during ion formation in an APCI or ESI source. See also nozzle–skimmer dissociation.
|
| |
|
Indium is a chemical element with chemical symbol In and atomic number 49. This rare, soft, malleable and easily fusible post-transition metal is chemically similar to aluminium or gallium but more closely resembles zinc (zinc ores are also the primary source of this metal).
Indium's current primary application is to form transparent electrodes from indium tin oxide in liquid crystal displays, and this use largely determines its global mining production. It is widely used in thin-films to form lubricated layers (during World War II it was widely used to coat bearings in high-performance aircraft). It is also used for making particularly low melting point alloys, and is a component in some lead-free solders. Radioactive indium-111 is used in nuclear medicine as a imaging agent to follow the movement of leukocytes in the body.
Source: Wikipedia
|
| |
|
The inductively coupled plasma (ICP) is the most widely used source for atomic emission and inorganic mass spectrometry. A "flame-like" plasma is sustained by means of a radiofrequency electric current via an induction coil (electrode-less) within a flowing plasma gas (mostly argon, but other gases such as Helium, Nitrogen or Air are principally possible). The plasma gas is transported into the discharge region via a quartz torch providing different channels structuring the gas-flow so that the high temperature plasma (ionised argon) does not melt the discharge container and the sample stream can be injected into the highly viscous plasma. The plasma with its very high temperature in the range of 7000-8000K efficiently desolvates, vaporizes, dissociates, atomizes, excites, and ionizes samples introduced as gases, vapors or aerosols. In this way, the ICP can be used as an atomizer and excitation source for atomic spectrometry (AES, AFS) or as an ion source for mass spectrometry ( ICP-MS).
|
| |
|
The act of swallowing something through eating, drinking, or mouthing
objects. A hazardous substance can enter the body this way
[see route of exposure].
Source: ATSDR Glossary of Terms
|
| |
|
The act of breathing. A hazardous substance can enter the body this way
[see route of exposure].
Source: ATSDR Glossary of Terms
|
| |
|
– No water molecule is present between the ion or molecule and the surface functional group to which it is bound, and the bonding is covalent or ionic. Inner-sphere complexes can be monodentate (metal is bonded to only one oxygen) or bidentate (metal is bonded to two oxygens) and mononuclear or binuclear. Inner-sphere complexation can increase, reduce, neutralize, or reverse the charge on the
sorptive regardless of the original charge. Adsorption of ions via inner-sphere complexation can occur on a surface regardless of the original charge. Inner-sphere complexation is usually slower than
outer-sphere complexation and is often not reversible.
|
| |
|
|
Sometimes referred to in short as inorganic arsenic, inorganic
arsenic compound contain arsenic (As) and at least one other element,
but no carbon (C). Inorganic arsenic exists in four main chemical forms
known as valency or oxidation states. Valency is a measure of the
ability of a compound to combine with other elements, such as hydrogen.
The dominant forms are:
- Arsenite, with a valency of 3, also referred to as trivalent
arsenic (As (III), As+3), and
- Arsenate, with a valency of 5, also referred to as pentavalent
arsenic (As (V), As+5
Inorganic arsenic (compounds) are mainly of geological origin and can be found
in groundwater used as drinking water in certain parts of the world.
|
| |
|
|
Includes elemental mercury and mercury bound to other inorganic molecules and compounds, including inorganic ligands and sulfides.
|
| |
|
|
Form of P that was not formed primarily by biological processes and is usually a collective term that refers to mineral forms of P such as compounds of either aluminum or iron in acidic media, or calcium in calcareous, alkaline media. This fraction generally represents the sum of OPO4 and acid hydrolyzable P, but some of the organic P maybe released as a result of acid hydrolysis.
|
| |
|
continuous incremental change over time in indication, due to changes in metrological
properties of a measuring instrument (VIM 4.21)
|
| |
|
A measure of instrument performance based on the instrumental signal-to-noise ratio or the variability of a standard blank sample. Unlike the limit of detection, it does not depend on the sample matrix, since it is measured in the absence of sample.
(see also method detection limit)
|
| |
|
An instrumental parameter is an aspect of the instrument which, if altered, may
change the output from the instrument.
|
| |
|
|
Correcting an analytical result for the contribution to the measured concentration by interelement interferences.
|
| |
|
instrumental analysis:
a device for coupling one instrument with a second instrument, for example, a chromatograph with an atomic spectrometer.
|
| |
|
|
Interference (in chemical analysis) is a systematic error in the measure of a signal caused by the presence of concomitants in a sample.
|
| |
|
Mass or concentration of interfering substance which gives the same measurement reading as unit mass or concentration of substance being measured.
|
| |
|
|
Any component of the sample affecting the final measurement.
|
| |
|
A study in which several laboratories measure a quantity in one or more ‘identical’ portions of homogenous, stable materials under documented conditions, the results of which are compiled into a single report.
|
| |
|
|
Series of measurements of one or more quantities performed independently by a number of laboratories on samples of a given material in order to gain information about the reliability and accuracy of a method or about the true quantities within the material.
|
| |
|
|
A material that is present or added to a sample, undergoing instrumental analysis, to serve as an intensity reference for to permit correction for inefficiencies. In order that the internal standard can be used for the correction of non-spectroscopic interferences, it should behave similar to the target analyte. Depending on the technique used (e.g. ICP-AES, ICP-MS) similarity can be expressed in terms of excitation energy, ionization energy or the sum of both.
|
| |
|
|
"The International Agency for Research on Cancer (IARC) is part of the World Health Organization (WHO).
IARC's
mission is to coordinate and conduct research on the causes of human
cancer, the mechanisms of carcinogenesis, and to develop scientific
strategies for cancer control. The Agency is involved in both
epidemiological and laboratory research and disseminates scientific
information through publications, meetings, courses, and fellowships."
It has collaborated to and published many highly recognized scientific publications.
Most publications are availaible from the webpage IARC Monographs Programme on the Evaluation of Carcinogenic Risks to humans  ,
"The IARC Monographs series publishes authoritative independent
assessments by international experts of the carcinogenic risks posed to
humans by a variety of agents, mixtures and exposures." ,
"The IARC Monographs series publishes authoritative independent
assessments by international experts of the carcinogenic risks posed to
humans by a variety of agents, mixtures and exposures."
IARC
distinguishes between four groups of compounds or physical factors
based on the existing scientific evidence for carcinogenicity: Standard IARC classification
(Source: IARC website )
|
| |
|
|
The International Programme on Chemical Safety (IPCS) was established in 1980 by the WHO, the UNEP and the ILO
(International Labour Organisation) "for the early warning and
prevention of harmful effects of chemicals to which humans were being
increasingly exposed, and for the assessment of the potential risks to
human health."
It has collaborated to and published many highly recognized scientific publications.
Most
publications are availaible from the INCHEM website www.inchem.org, "a
means of rapid access to internationally peer reviewed information on
chemicals commonly used throughout the world, which may also occur as
contaminants in the environment and food." Publications include:
|
| |
|
|
Water contained between the particles of soil or sediment.
|
| |
|
|
A radioactive isotope of iodine, a nonmetallic element of the halogen
group with an atomic mass of 123 and a half-life of 13.2 hours with
radioisotopic activity. Selectively accumulating in the thyroid tissue,
iodine I 123 emits gamma rays that can be detected with gamma
scintigraphy, allowing localization of thyroid tissue. This agent may
be used as a tracer in whole body scintigraphy (WBS) to localize
thyroid carcinoma metastases.
|
| |
|
|
A radioactive isotope of iodine, a nonmetallic element of the halogen
group, with an atomic mass of 124 and a half-life of 4.18 days with
radioisotopic activity. Selectively accumulating in thyroid tissue,
iodine I 124 emits positrons that can be detected by positron emission
tomography (PET), allowing localization of thyroid tissue. This
radiosiotope also emits gamma rays.
|
| |
|
|
A radioactive isotope of iodine, a nonmetallic element of the halogen
group. With a half-life of 60 days, iodine 125 occurs naturally and can
be produced artificially. This agent has both therapeutic and
diagnostic uses, particularly in thyroid disease.
|
| |
|
|
A radioactive isotope of iodine with an atomic mass of 131, a half life
of eight days, and potential antineoplastic activity. Selectively
accumulating in the thyroid gland, iodine I 131 emits beta and gamma
particles, thereby killing thyroid cells and decreasing thyroid hormone
production.
|
| |
|
|
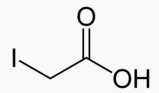 Iodoacetic acid is an organic compound with the chemical formula ICH2CO2H. It is a derivative of acetic acid. It is a toxic compound, because, like many alkyl halides, it is an alkylating agent.
|
| |
|
|
Iodothyronine deiodinase ( 1.97.1.10 ) (DI) is the
vertebrate enzyme responsible for the deiodination of the prohormone
thyroxine (T4 or 3,5,3',5'-tetraiodothyronine) into the biologically
active hormone T3 (3,5,3'-triiodothyronine) and of T3 into the inactive
metabolite T2 (3,3'-diiodothyronine). All known DI are proteins of
about 250 residues that contain a selenocysteine at their active site.
Three types of DI selenoenzymes are known, type II is essential for
providing the brain with the appropriate levels of T3 during the
critical period of development, and type III is essential for the
regulation of thyroid hormone inactivation during embryological
development.
|
| |
|
|
An iodated derivative of L-tyrosine. This is an early precursor to L-thyroxine, one of the primary thyroid hormones. Iodotyrosine is made from tyrosine via thyroid peroxidase and then further iodinated by this enzyme to make the di-iodo an tri-iodo variants. Two molecules of di-iodotyrosine combine to form T4, and one molecule of mono-iodotyrosine combines with one molecule of di-iodotyrosine to form T3.
|
| |
|
|
A collective term for any of a variety of techniques that involve irradiation of a sample with an ion beam for the purpose of analysis. Included are particle-induced gamma and x-ray emission, Rutherford backscattering spectroscopy, nuclear microprobes and other methods of nuclear reaction analysis.
|
| |
|
|
(IC) - An ion-exchange technique in which low concentrations of anions or cations are determined using low-capacity ion exchangers with weak buffers. Conductivity detectors are often used. Ion chromatography is practiced in two forms. In suppressed IC, a second column is used to remove the buffer ions so that sample ions can be more easily detected; membrane separator is sometimes used. In nonsuppressed IC, weakly conducting buffers at low concentration are carefully selected, and the entire effluent is passed through the detector; ions are detected above the background signal.
|
| |
|
An ion cyclotron resonance mass spectrometer (ICRMS) is a device for storage and mass analysis of ions. The ions are held in the cell by a combination of a static magnetic field and a coincident electrical field generated by potentials applied to all walls of the metal cell. Ions attain a coherent cyclotron orbit with frequency proportional to mass. Ions are detected by monitoring the alternating electrical current generated in detector plates by their regular orbits. A Fourier transformation converts the monitored frequency to ion mass.
  
|
| |
|
|
Ion desolvation is the removal of solvent molecules clustered around a
gas-phase ion by heating or collisions with inert gas molecule.
|
| |
|
- In surface chemistry, if the adsorption of one or several ionic species is accompanied by the silmultaneous desorption (displacement) of an equivalent amount of one or more other ionic species, this procedure is called ion exchange.
- The process of exchanging ions betweeen a solution and an ion exchanger
|
| |
|
|
An ion funnel is a device consisting of a series of stacked ring
electrodes with progressively decreasing inner diameter to which a
combined radio frequency and fixed voltage is applied.The resulting field focuses ions travelling along the central axis.
|
| |
|
|
A primary high-energy ion beam for SIMS which is designed to act on a small area of the target sample. Typical beam dimensions are in the tens of nanometres.
|
| |
|
The totality of ion focussing devices in a mass spectrometer. These are present in the source, interface from source to analyser, analyser(s), collision chambers and detector.
|
| |
|
|
An HPLC method in which ions are "paired" with special agents, thus neutralizing the ions and allowing them to be thus retained on a standard reversed-phase column.
|
| |
|
The part of the mass spectrometer used for sample ionization, since only particles which carry an electric charge can be analysed in a mass spectrometer. A variety of ion sources are in common use, each designed to ionize a specific class of atom or molecule.
See also: APCI, API, electron ionization (EI), chemical ionization, electrospray ionization, thermal ionization, plasma ionization etc.
|
| |
|
An ion trap analyzer consists of two end caps and a ring electrode assembled into a compact device that serves as a mass analyzer. The three-dimensional rotationally symmetric quadrupole field stores ions (externally generated) at its center. An additional electrical signal is then applied to mass-selectively eject ions to an external detector.
  
|
| |
|
|
(IEC) - a mode of liquid chromatography in which ionic substances are separated on cationic or anionic sites of the packing. The sample ion (and usually a counterion) will exchange with ions already on the ionogenic group of the packing. Retention is based on the affinity of different ions for the site and on a number of other solution parameters (pH, ionic strength, counterion type, etc.).
|
| |
|
A chemical interaction between a positive or negative ion and an uncharged molecule.
Source: IUPAC
|
| |
|
The interaction of a metal ion with a ligand where waters of hydration are retained. There is at least one H2O molecule ‘between” the ions interacting.
|
| |
|
|
(IPC) - form of liquid chromatography in which ions in solution can be "paired" or neutralized and separated as an ion pair on a reversed-phase column. Ion-pairing agents are usually ionic compounds that contain a hydrocarbon chain that imparts a certain hydrophobicity so that the ion pair can be retained on a reversed-phase column. Ion-pairing can also occur in normal-phase chromatography when one part of the pair is loaded onto a sorbent, but this technique is not as popular as the RPC technique.
|
| |
|
|
An ion/molecule reaction (I/M rxn) occurs between an ion and a neutral gas-phase molecule to cause ionization (as in protonation in chemical ionization), or changes in the internal energy of one or both of the reactants.
|
| |
|
|
The ionisation efficiency is the ratio of the number of ions formed to the number of electrons used.
|
| |
|
|
A matrix effect occurring in ion sources in which ionisation of an analyte is suppressed by the presence of a coeluting matrix component.
|
| |
|
|
ratio of the number of ions formed to the number of electrons, ions, or photons used in an ionization process
|
| |
|
The ionization energy is the minimum energy required to remove an electron from an atom or molecule in order to produce a positive ion.
  
|
| |
|
|
A salt of an alkali metal added to suppress ionization of an analyte. The alkali metal is easily ionized resulting in a flux of electrons which shift the ionization equilibrium of analyte towards the formation of atoms.
|
| |
|
|
(free) metal content of a cell
|
| |
|
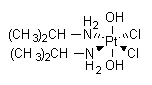 Iproplatin is a second generation metallodrug developed for cancer therapy. Iproplatin binds to and forms DNA crosslinks and platinum-DNA adducts,
resulting in DNA replication failure and cell death. Although less
prone to glutathione inactivation compared to cisplatin, resistance to
this agent has been observed in vitro due to repair of platination
damage by tumor cells.
IUPAC name: cis-dichlorobis(isopropylamine)-trans-dihydroxoplatinum(IV)
|
| |
|
|
A colloidal solution containing ferric oxyhydroxide complexed with
polymerized dextran, used as a form of parenteral iron-replacement
therapy. Upon administration and absorption, the iron dextran complex is
removed from plasma by the reticuloendothelial system which cleaves it
into the components iron and dextran; ferric iron subsequently binds to
transferrin or is stored as hemosiderin or ferritin. Transferrin-bound
iron is transported in the plasma to the liver, spleen and bone marrow,
where is it is incorporated into hemoglobin (Hgb) and to muscle where it
is incorporated into myoglobin (Mb). Use of this agent circumvents the
gastrointestinal adverse effects commonly encountered with the use of
orally administered iron salt preparations. Because of cross-reactivity with antibodies targeted against
polysaccharides similar to dextran, anaphylactic reactions may occur with
this type of iron formulation.
|
| |
|
In ICP-MS, the signal for a specific isotope of an element is often obscured by overlapping signals due to background gases or compounds producing isobaric ions of nearly identical m/z. A common problem arises with argon oxide and iron with m/z values of 55.9573 and 55.9354, respectively.
|
| |
|
Isobaric ions have identical masses (at whatever level of accuracy chosen) but have differen atomic compositions. A common example is a positive ion at m/z 28 which can have the empirical formulas of CO+, N2+ , or C2H4+.
  
|
| |
|
|
Refers to an HPLC separation where the mobile phase composition stays the same during the entire analysis. Isocratic separations are the opposite of gradient elution separations.
|
| |
|
|
Isokinetic sampling (in atmospheric chemistry) is a technique for collecting airborne particulate in which the sampling device has a collection efficiency of unity for all sizes of particles in sampled air, regardless of wind velocity and direction of the instrument. The air stream entering the collector has a velocity (speed and direction) equal to that of the air in the gas stream just ahead of the sampling port of the collector.
|
| |
|
|
Isomers are two or more chemical compounds with the same molecular formula but having different properties owing to a different arrangement of atoms within the molecule.
|
| |
|
|
Atoms of the same element (i.e. having the same atomic number) which differ in mass number are called isotopes of that element. The isotopes of an element are identical in chemical properties, and in all physical properties except those determined by the mass of the atom. The different isotopes of an element contain different numbers of neutrons in their nuclei. Nearly all elements found in nature are mixtures of several isotopes.
(Uvarov, E. B., d. R. Chapman, and A. Isaacs. A Dictionary of Science. Third Edition. Penguin Books Ltd., Harmondsworth, England, 1966.)
|
| |
|
|
When the change in the isotopic abundances of an element are a regular function of the mass
difference between the isotopes. Physical or chemical processes can induce such changes.
These changes may result from natural or anthropogenic processes and even from the
measurement process itself.. Both the mass spectrometer ion source, the detector and the
techniques used to prepare samples for mass spectrometry can introduce isotopic (mass)
fractionation.. The magnitude of this effect usually increases with increasing fractional mass
difference between the isotopes. Consequently the magnitude of the effect is greater for elements
with smaller atomic numbers.
|
| |
|
The substitution of one or more atoms in a molecule with a less abundant stable elemental isotope, commonly deuterium (2H), 13C, 15N or 18O.
|
| |
|
|
In isotope ratio mass spectrometry (IRMS), element isotope ratios are
determined
very accurately and precisely. Typically, single focusing
magnetic sector
mass spectrometers with fixed multiple detectors (one per
isotope) are
used. Complex compounds are reduced to simple molecules prior
to measurement,
for example, organic compounds are combusted to CO2,
H2O
and N2.
|
| |
|
The ICAT approach is based on affinity tags targeting of functional groups in proteins that are labeled
after the proteins are isolated.
|
| |
|
In order to establish the relevance of a protein to an organism’s phenotype, it is important to be able to determine quantitative changes in its expression. This can be achieved by labelling with a compound (tag) which contains stable isotopes such as 2H. One batch of the organism is tagged with the heavy form of the label, containing the stable isotopes (2H), and another batch is tagged with the light form, containing 1H. The reactions are specific for cysteine residues within the proteins. The labelled samples are mixed and digested with trypsin and the isolated peptides are analysed by LC/MS/MS, enabling the relative abundances of each peptide pair, containing the light and heavy label, to be determined. ICAT is a commercial term.
|
| |
|
|
Isotope-dilution analysis (IDA) is a kind of quantitative analysis based on the measurement of the isotopic abundance of a nuclide after isotope dilution by mixing with one or more of its isotopes with the test portion. In this way quantitative determinations can be performed by simply measuring isotope ratioes rather than absolute concentrations reducing the impact of non-quantitative analytical processes such as analyte extraction and separation or matrix interferences.
|
| |
|
|
The relative number of atoms of a particular isotope in a mixture of the isotopes of an element, expressed as a fraction of all the atoms of the element.
|
| |
|
A process by which the relative abundance of the isotopes of a given
element are altered, thus producing a form of the element that has been
enriched in one particular isotope and depleted in its other isotopic
forms.
|
| |
|
|
Isotopic patterns are important indications for the elemental composition of an ion. Sometimes they are referred to as isotopic distribution or incorrectly as isotopic cluster. If
metals with complicated isotopic patterns are contained in a molecule
the agreement of calculated
and experimental isotopic pattern is of the same importance for the
assignment of a molecular formula as the result from HR-MS.
|
| |
|
|
Is a material containing an element for which the isotopic composition and associated
uncertainties have been determined with small combined uncertainty. A sufficient amount of this
material should be available for widespread use.
|
| |
|
|
Indicator made of the same element as the one to be monitored, but having a different isotopic composition. Isotopic tracers make it possible to determine the provenance of certain molecules in a chemical compound and to monitor the movement of chemical substances on a large scale.
|
| |
|
|
Is a form of the chemical element having one or more isotope abundance artificially enhanced
compared with the natural element. The terms spike and tracer relate to applications of this
material.
|
| |
|