|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
|
Polyacrylamide gel electrophoresis (PAGE) is a technique for the seperation of proteins in which a polyacrylamide gel is used as a supporting matrix through which proteins can migrate and electrophoresis is used as the principle for separation. Separation occurs, because small molecules can manuver through the polyacrylamide gel faster than big molecules.
|
| |
|
The term parent ion is synonymous with the term precursor ion and denotes the ion that dissociates to a smaller fragment ion, usually as a result of collision-induced dissociation in an MS/MS experiment. Precursor ion is the preferred term.
  
|
| |
|
|
Administration of substance into organism not through gastrointestinal tract but through intramuscular, subcutaneous or intravenous injection
|
| |
|
|
The intravenous administration of nutrients, through a central or peripheral vein. This mode of nutrition is done to bypass the normal route of nutrition via the gastrointestinal tract when the intestinal tract is nonfunctional. This could be as simple as carbohydrate calories delivered as simple
sugar in an intravenous solution or all of the required nutrients (total parenteral nutrition) could
be delivered including carbohydrate, protein, fat, electrolytes (for
example sodium and potassium), vitamins and trace elements (for example
copper and zinc).
|
| |
|
|
A particle beam interface (PB) is a method for coupling liquid
chromatography to mass spectrometry in which the effluent is passed
through a heated capillary to form an expansion of vapor and aerosol
particles.
After passing through a skimmer, the beam impinges on a heated surface
to form ions through chemical ionization at the surface or ionization of
the resulting vapor in a CI or EI source.
|
| |
|
|
An ion beam technique particulary well suited for the determination of light mass element concentrations into materials. Anaylsis depth up to a few micrometers.
|
| |
|
|
The concentration of metals in the portion of a sample that is retained by a 0.45 µm filter.
|
| |
|
(referred to also as Total Suspended Phosphorus)
Phosphorus fraction that is adsorbed or absorbed on soil or sediment particles, and maybe comprised of both organic and mineral forms. This fraction is usually quantified by subtracting TDP from TP. Alternatively, this fraction can be measured by subjecting the particulates collected on 0.45 µm* to a rigid digestion procedure and analyzing the digestate for P (APHA, 1989).
|
| |
|
Separation based mainly on differences between the solubilities of the components in the stationary phase (gas chromatography), or on differences between the solubilities of the components in the mobile and stationary phases (liquid chromatography).
Source: IUPAC
|
| |
|
|
Concentration of a substance in one phase devided by the concentration of the substance in the other phase when the heterogeneous system of the phases is in equilibrium.
Note 1: The ratio of concentrations (or, strictly speaking, activities) of the same molecular species in the two phases is constant at constant temperature.
Note 2: The octanol/water partition coefficient is often used as a measure of the bioconcentration factor for modeling purposes.
Note 3: This term is in common usage in toxicology but is not recommended by IUPAC for use in chemistry and should not be used as a synonym for partition constant, partition ratio, or distribution ratio.
Measure of the sorption phenomenon, whereby a chemical compound is divided between the solid and the liquid phase; also referred to as adsorption partition coefficient.
|
| |
|
The ratio between the amount of substance attached to suspended matter per unit of mass and the amount of dissolved substance per unit of volume of water.
Source: IAEA (2000)
|
| |
|
|
Ratio of the concentration of a substance in a single definite form, A, in the extract to its concentration in the same form in the other phase at equilibrium, e.g. for an aqueous/organic system:
KD(A)= [A]org/[A]aq
|
| |
|
Sampling technique based on free flow of analyte molecules from the sampled medium to a receiving phase in a sampling device, as a result of a difference between the chemical potentials of the analyte in the two media. The net flow of analyte molecules from one medium to the other continues until equilibrium is established in the system, or until the sampling period is stopped. Sampling proceeds without the need for any energy sources other than this chemical potential
difference.
|
| |
|
|
procedure whereby a spectrum, generated by peak synthesis, is adjusted to match a measured spectrum
|
| |
|
A means to acquire a mass spectrum by jumping from one mass to charge to another;
measurements are made at the mass to charge of each mass spectral peak; only ions with mass-to-charge ratios of interest are measured, skipping mass-to-charge ratios that are not of interest.
|
| |
|
A cooling method based on the thermoelectric effect. It is largely employed to cool the sensitive light detectors (CCDs) as a solid-state replacement of much more technically demanding cooling
using liquid nitrogen.
|
| |
|
Penning ionization (PI) is caused by interactions between excited neutral atoms or molecules (A. or M.) and sample molecules. The internal energy of the excited neutral particles must exceed the ionization energy of the sample molecules and the lifespan of the excited state must be longer than the interac-tion time.
M+ A- --> M+. + A + e-
|
| |
|
In the experimental measurement of an exact mass, the percent accuracy is calculated as the (true mass - observed mass)/true mass x 100%. The percent accuracy is often expressed in parts per million (ppm). For example, 0.01% accuracy is 100 ppm. A 100 ppm accuracy for an ion with a mass of 1000 Da is 0.1 Da.
  
|
| |
|
Matrix of algae, microbes, and detritus attached to submerged surfaces in aquatic ecosystems.
|
| |
|
|
A permanganate is a chemical compound that contains the permanganate ion (MnO4-). Because manganese is in the +7 oxidation state, the permanganate ion is a strong oxidizer. Permanganates are salts of permanganic acid. Permanganate is a strong oxidizer, and similar to perchlorate. Being a strong oxidizer it is in common use in qualitative analysis experiments involving redox reactions (permanganometry).
|
| |
|
Chromatographic separation based mainly upon exclusion effects, such as differences in
molecular size and/or shape (e.g. molecular-sieve chromatography) or in charge (e.g. ion-exclusion chromatography). The term gel-permeation chromatography is widely used for the process when the stationary phase is a swollen gel. The term gel-filtration is not recommended.
|
| |
|
|
Attribute of a substance that describes the length of time that the substance remains in a particular environment before it is physically removed or chemically or biologically transformed.
|
| |
|
Inorganic substance that is stable in the environment, is liable to long-range transport, may bio-
accumulate in human and animal tissue, and may have significant impacts on human health and the
environment.
Note 1: Examples are arsenides, fluorides, cadmium salts, and lead salts.
Note 2: Some inorganic chemicals, like crocidolite asbestos, are persistent in almost all circumstances, but others, like metal sulfides, are persistent only in unreactive environments; sulfides can generate hydrogen sulfide in a reducing environment or sulfates and sulfuric acid in oxidizing environments. As with organic substances, persistence is often a function of environmental properties.
|
| |
|
|
Substance intended to kill pests: in common usage, any substance used for controlling, preventing, or destroying animal, microbiological or plant pests.
|
| |
|
The ultimate characterization of all of the chemical constituents of petroleum, along with their interactions and reactivity.
|
| |
|
|
The pulsed flame photometric detector (PFPD) is based on a pulsed flame for the generation of flame chemiluminescence. The detector used for gas chromatography operates with a fuel-rich mixture of hydrogen and air that is ignited 3-4-times per second . The flame propagates in counterdirection of the gas-flow into a combuston chamber, where the flame extinguishes. Carbon light emissions and the emissions from the hydrogen/oxygen combustion gases are complete within 2-3 ms, after which a number of heteroatomic species give delayed emissions lasting up to 4-20 ms. These delayed emissions are filtered with a wide bandpass filter, detected by an appropriate photomultiplier tube (PMT). and electronically gated to eliminate background flame emission. The PFPD can detect at least 28 elements, 13 of them (S, P, N, As, Se, Sn, Ge, Ga, Sb, Te, Br, Cu and In) with high selectivity.
|
| |
|
|
A volume of space, solid, liquid, or gas in equilibrium with other volumes and described by a boundary. A homogeneous, distinct portion of a chemical system.
|
| |
|
|
Phase-system switching is an LC/MS technique in which the analyte
emerging from the LC column is transferred to a trapping column where
the solvent and buffer is washed off. The analyte is then dissolved in a
solvent that is compatible with the LC/MS interface for ionization
|
| |
|
|
Strictly speaking, phenylmercury is a cation,
C6H5Hg+, that forms organic mercury
compounds such as phenylmercury acetate and phenylmercury chloride, which have
both been used as fungicides. The term 'phenylmercury' is sometimes used as a
generic term to describe phenylmercury compounds.
|
| |
|
|
The part of the proteome modified by phorphorylation (a post-translational modification)
|
| |
|
Phosphorus is one of the key elements necessary for growth of plants and animals. Phosphates (PO4---) are formed from this element. Phosphates exist in three forms: orthophosphate, metaphosphate (or polyphosphate) and organically bound phosphate. Each compound contains phosphorous in a different chemical formula. Ortho forms are produced by natural processes and are found in sewage. Poly forms are used for treating boiler waters and in detergents. In water, they change into the ortho form. Organic phosphates are important in nature. Their occurrence may result from the breakdown of organic pesticides, which contain phosphates. They may exist in solution, as particles, loose fragments or in the bodies of aquatic organisms. Rainfall can cause varying amounts of phosphates to wash from farm soils into nearby waterways. Phosphate will stimulate the growth of plankton and aquatic plants which provide food for fish. This may cause an increase in the fish population and improve the overall water quality. However, if an excess of phosphate enters the waterway, algae and aquatic plants will grow wildly, choke up the waterway and use up large amounts of oxygen. This condition is known as eutrophication or over-fertilization of receiving waters. This rapid growth of aquatic vegetation eventually dies and as it decays it uses up oxygen. This process in turn causes the death of aquatic life because of the further reduction of dissolved oxygen levels.
|
| |
|
|
A process involving the transfer of a phosphate group (catalyzed by enzymes) from a donor to a suitable acceptor; in general an ester linkage is formed, for example:
ATP + alcohol → ADP + phosphate ester
|
| |
|
|
chemical reactions induced by the presence of ultra-violet or visible light.
|
| |
|
Ionization resulting from the interaction of a photon with any particle which is in consequence ionized.
see for example: APPI (Atmospheric pressure photo ionization)
Source: IUPAC
|
| |
|
A light-induced bond cleavage. The term is often used incorrectly to describe irradiation of a sample.
|
| |
|
A device to prevent photons (light) reaching an ion detector.
(see also: off-axis detector)
|
| |
|
|
A specific instance of bioavailability with reference to plants. In some instances it is useful to differentiate between phytoavailability and bioavailability along the food chain. Phytoavailability controls the transfer of a trace element from soil to a plant, and bioavailabilty controls the transfer of the trace element from the plant material to the receptor organism, the transfer factors are unlikely to be the same.
|
| |
|
Phytochelatins (PCs) are oligomers of glutathione, produced by the enzyme phytochelatin synthase (PCS). They are found in plants, fungi, nematodes and all groups of algae including cyanobacteria and are important for heavy metal detoxification.
PCs have the general structure of (g-glutamyl-cysteinyl)n-glycine (n=2-11) and are therefore abbreviated as PC2 to PC11.
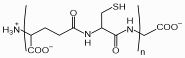
Chemical structure of phytochelatin with n=2-11
Phytochelatins act as chelators, binding metal ions through thiol coordination and by this way,
limit damage to metabolic processes in reducing the cytotoxic free metal
ion. Because phytochelatin synthase uses glutathione with a blocked thiol group in the synthesis of phytochelatin, the presence of heavy metal ions that bind to glutathione causes the enzyme to work faster. Therefore the amount of phytochelatin increases when the cell needs more phytochelatin to survive in an environment with high concentrations of metal ions. Its widespread presence in microorganisms suggests that PC-based metal detoxification might be an ancient type of defense mechanism established in micro-algae or micro-fungi.
|
| |
|
|
use of plants to remove contaminants from soils into plant roots or shoots.
|
| |
|
|
The use of living plants, plant parts, or plant extracts to treat contaminated sites. Certain plants have the ability to bioaccumulate toxic elements and thereby detoxify their surroundings.
|
| |
|
|
Organic compounds released by the roots of some plants suffering from a deficiency or iron or certain other micronutrients. They mobilize iron and elements coprecipitated onto iron oxides and render them available for uptake by the plant
|
| |
|
|
use of plants to reduce the bioavailability of pollutants in the environment.
|
| |
|
|
use of plants to volatilize pollutants.
|
| |
|
|
A new generation organic platinum analog with an extended spectrum of
antineoplastic activity. Designed to overcome platinum drug resistance,
picoplatin alkylates DNA, forming both inter- and intra-strand
cross-linkages, resulting in inhibition of DNA replication and
transcription, and the induction of apoptosis.
|
| |
|
|
Particle-Induced X-ray Emission Analysis (PIXE) is an analytical technique based on the creation of inner-shell vacancies in the atoms of the specimen by the bombardement with heavy charged particles (i.e. protons, alpha-particles, or heavy ions).
|
| |
|
|
(in biology)
1. Fluid component of blood in which the blood cells and platelets are suspended (blood plasma).
2. Fluid component of semen produced by the accessory glands, the seminal vesicles, the prostate, and in bulbo-urethral glands.
3. Cell substance outside the nucleus, i.e. the cytoplasm.
(in physics)
Gas, ionized to a certain degree
|
| |
|
|
Plasma desorption (PD) is one method of HIID. The high energy disintegration particles of 106Tc22+ and 142Ba18+ (~100MeV) from the radioactive 252Cf nucleus are directed at the back of a thin metal sheet or cellu-lose membrane spread with sample. Various ions are then created by desorption.
|
| |
|
The precious metals Ruthenium (Ru), Osmium (Os), Rhodium (Rh), Iridium (Ir), Palladium (Pd) and Platinum (Pt) are often called the "Platinum metals" or "Platinum group elements" (PGE) or "Platinum group metals" (PGM). The terms “noble” and “precious” metals are used to cover PGM and Gold.
|
| |
|
|
PM10: particulate matter which is less than 10
micrometres in diameter (a micrometer is 1 millionth of a metre); also
known as respirable particles.
|
| |
|
|
(Particulate matter less than 2.5 microns) Tiny solid or liquid particles, generally soot and aerosols. The size of the particles (2.5 microns or smaller, about 0.0001 inches or less) allows them to easily enter the air sacs deep in the lungs where they may cause adverse health effects; PM2.5 also causes visibility reduction.
|
| |
|
|
The endpoint used for contaminants with no cumulative properties. Its value represents permissible human exposure as a result of the natural occurrence of the substance in food and in drinking-water. In the case of trace elements that are both essential nutrients and unavoidable constituents of food, a range is expressed, the lower value representing the level of essentiality and the upper value the PMTDI.
|
| |
|
|
Pneumatically-assisted electrospray is a form of electrospray in which
the formation of droplets in the liquid stream is assisted by a gas flow
thereby allowing operation at higher liquid flow rates
|
| |
|
|
Statistical distribution specific for phenomena like photon time arrival at the detector, or noise distribution in the detector.
|
| |
|
|
A classical electroanalytical technique discovered in 1922 by J. Heyrovsky,
for which he was awarded the Nobel Prize for Chemistry in 1959. Essentially, it
is linear-sweep voltammetry using a dropping-mercury electrode for working
electrode and a large mercury pool as counter electrode.
|
| |
|
Any material entering the environment that has undesired effects.
|
| |
|
|
A molecular ion (an ion composed of more than one atom) that arises in the plasma or during ion extraction, and can appear at the same nominal mass as analyte ions. In ICP-MS polyatomics are usually interferences (such as ArO+).
|
| |
|
|
A very large molecule comprising a chain of many similar or identical molecular sub units (monomers) joined together (polymerised). An example is the polymer glycogen, formed from linked molecules of the monomer glucose
|
| |
|
in mineralogy - the formation of small multinuclear inorganic species such as dimers or trimers on a
surface.
|
| |
|
|
Polyoxometalates are large inorganic anions formed via condensation-addition reactions of simple oxoanions of early transition elements such as V, Mo and W.
e.g. 7 MoO42- + 8 H+ = [Mo7O24]6- + 4 H2O an isopolymolybdate 12 WO42- + SiO32- + 22 H+ = [SiW12O40]4- + 11 H2O a heteropolytungstate
|
| |
|
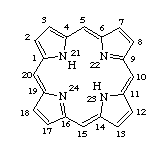 A macrocyclic molecule that contains four pyrrole rings linked together by single carbon atom bridges between the alpha positions of the pyrrole rings. Porphyrins usually occur in their dianionic form coordinated to a metal ion (e.g. V, Ni, Fe, Ga, Ti) in thermally stable complexes. Metalloporphyrins are ubiquitously present in natural energy resources, such as shale oil or coal. Their fate becomes of concern during burning and processing of these materials.
|
| |
|
|
With MALDI, this term designates the phenomenon occurring when the excessive internal energy of ions themselves or collisions with free gas cause dissociation immediately after the ions generated by laser illumination exit the high speed field region. Since the ions decay after leaving the ion source but before reaching the collector, this term corresponds to metastable dissociation.
|
| |
|
Postsource decay (PSD) processes are ion dissociations that occur within the drift region of a time-of-flight mass analyzer. After initial ion formation and acceleration out of the sourc into the flight tube, ions may dissociate or neutralize. In a linear time-of-flight instrument, these fragment ions and neutral species reach the detector at the same time as the precursor ions from which they are formed. However, these reactions can be specifically studied by using a reflectron (adjusting the ratio of accelerating to reflectron voltages in a stepwise manner) to bring fragment ions formed in post- source decay processes into focus at the detector.
  
|
| |
|
|
Potassium hexachloroplatinate is the inorganic compound with the formula K2PtCl6.
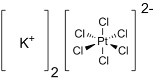
It is a yellow solid that is an example of a comparatively
insoluble potassium salt. The salt features the hexachloroplatinate(IV)
dianion, which has octahedral coordination geometry.It is a raw material for the synthesis of Pt-based drugs.
|
| |
|
|
Potassium tetrachloroplatinate(II) is the chemical compound with the formula K2PtCl4.
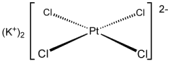
This reddish orange salt is an important reagent for the preparation of other coordination complexes of platinum. It consists of potassium cations and the square planar dianion PtCl42−.
It is a raw material for the synthesis of Pt-based drugs such as cis-platin.
|
| |
|
Approach to risk management that can be applied in circumstances of scientific uncertainty, reflecting a perceived need to take action in the face of a potentially serious risk without waiting for definitive results of scientific research.
|
| |
|
|
Process causing a solid to settle out of solution by the action of gravity or by a chemical reaction; a reaction betwen a soluble target substance and an added precipitant, resulting in the formation of a less soluble substances (known as a precipitate) that separates, in solid particles from a liquid.
|
| |
|
|
Precision is a term belonging to the concept of accuracy and is defined as the closeness of agreement between the results obtained by applying the same experimental procedure several times under prescribed conditions.
Source: International Vocabulary of Basic and General Terms in Metrology, International Standardization Organisation, Geneva, Switzerland, 2nd edition, 1993
|
| |
|
|
analytical procedure to increase the concentration of analytes in the analytical solution in comparison with that present in the original sample. The techniques used include cryofocussing, gas-solid extraction, solid-phase extraction (SPE), solid-phase microextrraction (SPME), solvent extraction and evaporation.
|
| |
|
|
Precursor Compounds that change chemically or physically after being emitted into the air and eventually produce air pollutants. For example, sulfur and nitrogen oxides are precursors for particulate matter.
|
| |
|
In an MS/MS experiment,mass-selected precursor ions are induced to dissociate into product ions, which are then mass analyzed by a second analyzer. The three common scans in the single-step MS/MS experiment are: product ion scan, precursor ion scan, and then the constant neutral loss scan. In the precursor ion scan, the second mass analyzer is set at the mass of the selected product ion, and then the first mass analyzer is scanned from that mass upwards. The result is a mass spectrum that contains signals for all the precursor ions that dissociate to that selected product ion.
  
|
| |
|
The predicted no effect concentration (PNEC) is an estimate of the
concentration of a potential hazardous substance for which no harmfull
effect is expected. This value is obtained from toxicological
dose-response data by applying uncertainty factors appropriate to the
uncertainty of the available data.
|
| |
|
MS:
In QIT mass spectrometry when automatic
gain control is active a prescan preceeds every analytical scan to
determine the proper analytical ion injection time. It consists of a
short (<10 ms) ion injection to determine the ion current.
AES:
In atomic emission spectrometry with CCD/CID detection systems with limited linear working range a prescan preceeds every analytical measurement to determine the proper exposure time for every channel in order to stay within the upper electron capacity of the detector element.
|
| |
|
|
Standard that is designated or widely acknowledged as having the highest metrological qualities and whose value is accepted without reference to other standards of the same quantity, within a specified context.
|
| |
|
A mathematical procedure for reducing the complexity of multidimensional data sets, while retaining the majority of the original variability. The first principal component accounts for the maximum variability possible and each successive component accounts for as much of the remaining variability as possible. The technique is used in chemometrics to visualise patterns within the data and is used in pattern recognition.
|
| |
|
|
The isotope of any given element with the highest relative abundance.
|
| |
|
In an MS/MS experiment, mass-selected precursor ions are induced to dissociate into product ions, which are then mass analyzed by a second analyzer. The three common scans in the single-step MS/MS experiment are: product ion scan, precursor ion scan, and then the constant neutral loss scan. In the product ion scan, the first mass analyzer is set at the mass of the selected precursor ion, and then the second mass analyzer is scanned from that mass downwards. The result is a mass spectrum that contains signals for all the product ions formed from that selected precursor ion.
  
|
| |
|
|
“A specimen containing analytes of
unknown concentration or identification that is sent to laboratories
participating in testing programs in order to independently verify the
laboratory technical competency.” [CLSI]
|
| |
|
Any laboratory testing program where stable specimen are sent to participating laboratories for analysis. Results from all participating laboratories are compared, pooled, and tabulated by the testing program operator with the purpose of improving laboratory performance.
  
|
| |
|
Propylation is a derivatization technique in which the analyte species
is transformed by adding propyl groups. For this alkylation either a
Grignard reagent based on propylmagnesium or sodium tetrapropylborate
can be used. Propylation is especially usefull for the analysis of
methyl- or ethyl-species, for which methylation or ethylation would
destroy molecular information.
|
| |
|
|
The chemical building blocks from which mammalian cells, organs, and tissues like muscle are made. Proteins also serve double-duty as hormones, enzymes and antibodies, which help fight off invading germs. Proteins are made of long chains of even smaller building blocks called amino acids. Amino acids determine the size, shape, and length of protein molecules. They also give protein molecules the odd ability to coil and uncoil like tiny, cellular snakes.
|
| |
|
The entire protein complement of a given genome, that is, the entirety of the proteins that are
expressed by the genome
|
| |
|
Study of the proteome of an organism. The term is most commonly associated with the use of
MS to identifiy proteins expressed in a given cell type or tissue under a given set of conditions
|
| |
|
|
When protons move between molecules or molecular ions to create
protonated molecules or multiply charged protonated molecules, the
particle giving the proton is called the proton acceptor. In proton
movement between molecules the proton donor is the deprotonated molecule
[M-H]-. Brönstead acids are proton donors.
|
| |
|
A protonated molecule is (usually) an ion formed by addition of a proton to the neutral molecule M , namely (M+H)+. The process of chemical ionization using a reagent gas such as methane forms such a protonated molecule. The transfer of a proton from one molecule to the other in the gas phase is an acid/base reaction in which the relative proton affinities of the reacting species describe the energetics of the reaction. Protonated molecules formed in other ionization sources (such as fast atom bombardment, electro-spray ionization, or MALDI) may not be the end result of such well-defined acid/base reactions. The term protonated molecular ion has been used to describe (M+H)+ but is usually discouraged.
  
|
| |
|
|
- a moving phase that acts as a stationary phase, e.g., the micelles in MEKC, which cause separation by a similar partitioning mechanism as a stationary phase in HPLC, but are not stationary.
|
| |
|
|
An endpoint used for a food contaminant with cumulative properties that has a very long half-life in the human body. Its value represents permissible human monthly exposure to a contaminant unavoidably associated with otherwise wholesome and nutritious foods.
|
| |
|
|
PTWI stands for Provisional Tolerable Weekly Intake. It describes the amount of a substance that can be eaten every week throughout a person`s life with no risk of negative health effects. Use of the word ”provisional” means that the assessment is temporary and that it will be reconsidered when more data is available. An assessment of this kind will always include a certain safety margin. Consuming more than the PTWI limit does not pose an immediate health risk, but the safety margin is reduced.
|
| |
|
|
The most sensitive ion detection method, in which each ion striking the detector creates an individual pulse. The count rate is limited by the recovery time (dead time) of the detector between pulses but is usually in the range from 1 to 106 counts per second for electron multipliers.
|
| |
|
|
A concentration technique for volatile solutes. Sample is purged with an inert gas that entrains volatile components onto an adsorptive trap. The trap then is heated to desorb trapped components into a GC column.
|
| |
|
|
Purity is defined as the fraction of the named material present in the stated chemical form. When using standards (including reference materials) it is obviously important to know what their composition is in terms of their purity.
|
| |
|
|
the transformation of a substance into another compound or compounds by the application of heat as the energy source.
|
| |
|
|
Sample is pyrolyzed (decomposed) in the inlet before GC analysis.
|
| |
|
In a pyrolysis source interfaced with a mass spectrometer, the sample is thermally decomposed in a reproducible pyrolysis. The gaseous products formed are then analyzed either as a mixture by mass spectrometry, or are analyzed by GC/MS. Pyrolysis mass spectrometry can be used for the analysis of otherwise nonvolatile samples.
  
|
| |
|