A group of Czech researchers proposes a novel LC-ICP-MS based approach for the accurate and precise chromium speciation in biological tissues. Obtained results indicated that the majority of chromium is bound to the solid residue obtained by leaching the tissue. Cr(VI) was determined in none of the samples investigated.
Background:The most significant chromium species in the biological environment are trivalent (Cr(III)) and hexavalent (Cr(VI)) states, differing in toxicity. While a low level of Cr(III) is considered to be beneficial for humans supporting the glucose metabolism, Cr(VI) is very toxic and even classified as mutagen and carcinogen. To make the situation even more complex, the occurrence and species distribution is dependent on various chemical factors such as pH values, redox potential and presence of oxidizing/reducing agents. The biological environment promotes the species interconversion and makes chromium speciation challenging. In view of these issues, chromium speciation in biological samples has been studied much less in comparison to environmental samples such as water, soils and sediments.
The new study:A group of Czech researchers now developed a method based on ion-exchange chromatography hyphenated with ICP-MS (IE-HPLC-ICP-MS) for the determination of soluble Cr(III) and total Cr(VI) species in tissue samples. The IE-HPLC-ICP-MS method is based on efficient alkaline extraction and was validated according to U.S. FDA guidelines for the validation of bioanalytical methods using two certified reference materials of hepatopancreas tissue. The accuracy of the speciation was further supported by results from the validated total chromium determination, which was used for chromium mass balancing.
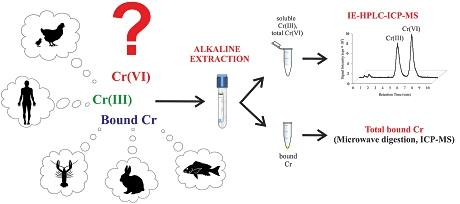
Chromatographic separation was based on a strong anion exchange column (PRP-X100, 150 x 2.1 mm, 5 µm) and a mobile phase composed of 30 mmol L−1 NH4NO3 in ultrapure water (pH 6) under isocratic conditions. ICP-MS detection was based on interference reduction by using the collision cell technology with helium as collision gas.
Chicken muscle tissue was used as the biological materials and was extracted with EDTA solution with pH 10.5. For mass balancing, the total chromium was determined in the original chicken tissue, and also in the residue after extraction and the supernatant.
Finally, the whole ICP-MS-based methodology was applied to the analyses of two certified reference materials of hepatopancreas tissue. Obtained results indicated that the majority of chromium in biological tissues is bound to the solid residue, Cr(VI) was determined in none of the samples investigated. The authors also observed, that Cr(III) was stable during all steps of the developed analytical approach, while about 30% of added Cr(VI) was reduced in biological tissues due to the presence of reducing agents in the sample matrix. They concluded that, based on this result, it is very unlikely that Cr(VI) exists in studied biological tissues.
Comment: The observation, that the strong oxidizing agent Cr(VI) gets reduced in presence of biological material, is nothing new and has been reported many times. Because of that, we at EVISA always questioned analytical results reporting Cr(VI) in biological materials and also never offered the analysis of Cr(VI) in such materials. Because of the chemistry of Cr(VI), the presence of Cr(VI) in biological materials can only be a transient effect. Therefore, reported Cr(VI) levels found in such materials are most probably only related to inadequate selectivity of the speciation analysis performed or artefacts of inadequate sample preparation. Speciation methods based solely on the "selective" extraction of Cr(VI) are known to lack adequate selectivity. Methodology to avoid errors by species interconversion during analysis is available (species specific isotope dilution analysis) but seldom being applied in such analysis. By knowing these facts, reviewers should reject reports relying on such inadequate methodology.
Michael Sperling
 The original study:
The original study:

Radka Pechancová, Jiří Gallo, David Milde, Tomáš Pluháček,
Ion-exchange HPLC-ICP-MS: A new window to chromium speciation in biological tissues, Talanta, 218 (2020) 121150.
DOI: 10.1016/j.talanta.2020.121150
 Related studies (newest first)
Related studies (newest first)
 Radmila Milacic
Radmila Milacic,
Janez Scancar,
Cr speciation in foodstuffs, biological and environmental samples: Methodological approaches and analytical challenges - A critical review, Trends Anal. Chem., 127 (2020) 115888.
DOI: 10.1016/j.trac.2020.115888
R. Pechancova, T. Pluhacek, D. Milde,
Recent advances in chromium speciation in biological samples, Spectrochim. Acta Part B, 152 (2019) 109–122.
DOI: 10.1016/j.sab.2018.12.008.
A. Akhtar, T.G. Kazi, H.I. Afridi, M. Khan, M. Bilal, N. Khan,
Application of modified cloud point extraction method for the chromium speciation in artificial saliva extracts of different snuff products, J. Ind. Eng. Chem., 59 (2018) 320–327.
DOI: 10.1016/j.jiec.2017.10.038.

R. Pechancová, T. Pluháček, J. Gallo, D. Milde,
Study of chromium species release in blood and joint effusion utilizing HPLC-ICP-MS, Talanta, 185 (2018) 370–375.
DOI: 10.1016/j.talanta.2018.03.100.
E.M. Hamilton, S.D. Young, E.H. Bailey, M.J. Watts,
Chromium speciation in foodstuffs: a review, Food Chem., 250 (2018) 105–112.
DOI: 10.1016/j.foodchem.2018.01.016.
 F. Seby
F. Seby,
V. Vacchina,
Critical assessment of hexavalent chromium species from different solid environmental, industrial and food matrices, Trends Analyt. Chem., 104 (2018) 54–68.
DOI: 10.1016/j.trac.2017.11.019.

B. Finley, P.K. Scott, M.E. Glynn, D. Paustenbach, E. Donovan, K.A. Thuett,
Chromium speciation in the blood of metal-on-metal hip implant patients, Toxicol. Environ. Chem. 99 (2017) 48–64.
DOI: 10.1080/02772248.2016.1148904.

A. Di Laura, P.D. Quinn, V.C. Panagiotopoulou, H.S. Hothi, J. Henckel, J.J. Powell, F. Berisha, F. Amary, J.F.W. Mosselmans, J.A. Skinner, A.J. Hart,
The chemical form of metal species released from corroded taper junctions of hip implants: synchrotron analysis of patient tissue, Sci. Rep. 7 (2017) 10952.
DOI: 10.1038/s41598-017-11225-w.

K. Pyrzynska,
Chromium redox speciation in food samples, Turk. J. Chem., 40 (2016) 894–905.
DOI: 10.3906/kim-1606-5.

Hamid Shirkhanloo, Mehri Ghazaghi, Hassan Z. Mousavi,
Chromium speciation in human blood samples based on acetylcysteine by dispersive liquid–liquid biomicroextraction and in-vitro evaluation of acetyl cysteine/cysteine for decreasing of hexavalent chromium concentration, J. Pharm. Biomed. Anal., 118 (2016) 1–8.
DOI: 10.1016/j.jpba.2015.10.018
K.M. Shah, P.D. Quinn, A. Gartland, J.M. Wilkinson,
Understanding the tissue effects of tribo-corrosion: uptake, distribution, and speciation of cobalt and chromium in human bone cells, J. Orthop. Res. 33 (2015) 114–121.
DOI: 10.1002/jor.22729.
 V. Vacchina
V. Vacchina, I. de la Calle,
F. Seby,
Cr(VI) speciation in foods by HPLC-ICP-MS: investigation of Cr(VI)/food interactions by size exclusion and Cr(VI) determination and stability by ion-exchange on-line separations, Anal. Bioanal. Chem., 407 (2015) 3831–3839.
DOI: 10.1007/s00216-015-8616-3.

B. Novotnik, T. Zuliani,
J. Scancar, R. Milacic,
Content of trace elements and chromium speciation in Neem powder and tea infusions, J. Trace Elem. Med. Biol., 31 (2015) 98–106.
DOI: 10.1016/j.jtemb.2015.04.003.

B. Novotnik, T. Zuliani,
J. Scancar, R. Milacic,
Chromate in food samples: an artefact of wrongly applied analytical methodology? J. Anal. Atom. Spectrom., 28 (2013) 558–566.
DOI: 10.1039/c3ja30233d.

A.J. Hart, P.D. Quinn, B. Sampson, A. Sandison, K.D. Atkinson, J.A. Skinner, J.J. Powell, J.F.W. Mosselmans,
The chemical form of metallic debris in tissues surrounding metal-on-metal hips with unexplained failure, Acta Biomater. 6 (2010) 4439–4446.
DOI: 10.1016/j.actbio.2010.06.006.
M.E. Soares, E. Vieira, M.D. Bastos,
Chromium speciation analysis in bread samples, J. Agric. Food Chem. 58 (2010) 1366–1370.
DOI: 10.1021/jf903118v.

H.J. Wang, X.M. Du, M. Wang, T.C. Wang, O.Y. Hong, B. Wang, M.T. Zhu, Y. Wang, G. Jia, W.Y. Feng,
Using ion-pair reversed-phase HPLC ICP-MS to simultaneously determine Cr(III) and Cr(VI) in urine of chromate workers, Talanta 81 (2010) 1856–1860.
DOI: 10.1016/j.talanta.2010.03.059.
S. Doker, S. Mounicou, M. Dogan,
R. Lobinski,
Probing the metal-homeostatis effects of the administration of chromium(VI) to mice by ICP MS and size-exclusion chromatography-ICP MS, Metallomics, 2 (2010) 549–555.
DOI: 10.1039/c004508j.

A.J. Hart, A. Sandison, P. Quinn, B. Sampson, K.D. Atkinson, J.A. Skinner, A. Goode, J.J. Powell, J.F.W. Mosselmans,
Microfocus study of metal distribution and speciation in tissue extracted from revised metal on metal hip implants, 14th International Conference on X-Ray Absorption Fine Structure, J. Phys. Conf. Ser. 190 (2009) 012208.
DOI: 10.1088/1742-6596/190/1/012208.
A.K. Das, R. Chakraborty, M.L. Cervera, M. de la Guardia,
Metal speciation in biological fluids - a review, Microchim. Acta, 122 (1996) 209–246.
DOI: 10.1007/bf01245784.
last time modified: September 21, 2024