A group of Nordic researchers investigated the Cu(II) binding to different proteins in human blood.
Background:Copper is an essential element as a cofactor for more than twenty metalloproteins that play important roles in cellular energy production, antioxidative defense and oxidative metabolism. For this reason, copper metabolism is strictly regulated, and its disturbance are characteristic for many diseases. Deviation from copper homeostasis is the primary cause of Wilson's and Menkes diseases and is involved in progression of inflammation, cancer, atherosclerosis and in many neurodegenerative diseases. The extracellular copper pool in the body is stored, transported and distributed in the body mainly by blood. While the structure and functioning of major copper proteins present in blood have been intensively studied for many decades, information about their metal-binding properties is still limited and often inconsistent.
The new study:The aim of the study cited here was to estimate the Cu(II)-binding affinities of major serum copper proteins (HSA, CP and α2M) in comparison with LMW Cu(II)-binding reference ligands including His by using a unified and direct approach, which gives thermodynamic background for understanding the distribution copper in the blood. For this purpose, the researchers have elaborated an LC-ICP-MS based approach for direct detection of Cu•Protein complex in the presence of metal-competing LMW Cu(II)-binding ligands. To cover the wide range of metal-binding affinities of different proteins, high-affinity DTPA and EDTA, intermediate affinity NTA and low-affinity His were used in titrations. To ensure that thermodynamic equilibrium was reached, the researchers also studied the kinetics of the metal release from the Cu-Protein complex.
The following proteins were investigated:
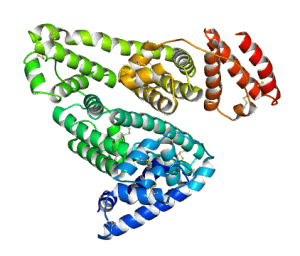
Structure of human serum albumin
|
Human serum albumin (HSA) is the most abundant protein in the blood. With an average concentration of 650 μM in serum, it constitutes more than 50% of serum proteins. HSA (MW 66.5 kD) is a monomeric multicargo transport and storage protein containing seven hydrophobic binding pockets for organic molecules such as fatty acids, metabolites, hormones and drugs. In vitro studies have also identified four distinct metal binding sites in HSA, differing in structure and metal-binding specificity.
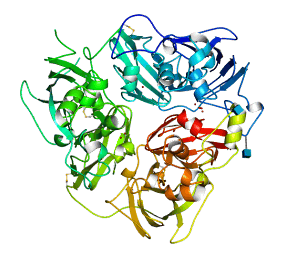
Structure of CP
|
Ceroluplasmin (CP) is a monomeric glycoprotein with MW of 132 kD and its average concentration in blood serum is 1.9 μM. CP is metallated in the secretory pathway, with six Cu ions bound in a highly cooperative manner. It is known that the cupric ions bound to CP are “not exchangeable”2, which seriously complicates determination of the Cu(II)-binding affinity of CP.
Structure of alpha-2-macroglobulin
|
Alpha-2 macroglobulin (α2M) is a homotetrameric glycoprotein with MW of 720 kD responsible for binding and inactivation of proteases. The average concentration of α2M in human plasma is 1.3 μM and there is evidence that α2M can bind copper ions and participate in copper delivery into mammalian cells.
The high and low-molecular weight (HMW and LMW) copper pools were separated by size-exclusion chromatography (SEC). Using a 1 mL Sephadex G25 Superfine column and a flow rate of 0.4 ml/min, this separation was accomplished within 4 minutes. The column materials was found to have some affinity for "free" Cu(II) ions, that was sufficiently lower as that for the ligands used in the titration experiment. To clean the column before each LC-ICP-MS experiment, column decoppering by injection of an EDTA solution was performed.
The average total concentration of copper in normal human blood serum is 16.7 μM. The results obtained by the researchers suggest that copper is distributed only between two principal serum proteins – CP and HSA.
Approximately 75% of the total copper pool is bound to the protein interior of CP in a way making them kinetically inert and practically non-exchangeable under physiological conditions, even in the presence of powerful chelating ligands like EDTA and DPA in great excess.
HSA is binding about 25% of the copper pool in a reversible way with however slow exchange rate. This copper pool could also be in equilibrium with Cu(II) ions bound to free amino acids, primarily His, depending on the HSA concentration.
Binding of copper to alpha-2 macroglobulin has not been observed by the researchers. The authors concluded that their results allow detection of disturbances in copper metabolism, characteristic for many diseases, and provide a rationale for effective metalloregulation.
 The original study:
The original study:

Tiina Kirsipuu, Anna Zadorožnaja, Julia Smirnova, Merlin Friedemann, Thomas Plitz, Vello Tőugu, Peep Palumaa,
Copper(II)-binding equilibria in human blood, Sci. Rep., 10 (2020) 5686.
DOI: 10.1038/s41598-020-62560-4
 Used techniques and instrumentation:
Used techniques and instrumentation:
 Related studies (newest first)
Related studies (newest first)

S. Catalani, M. Paganelli, M.EW. Gilberti, L. Rozzini, F. Lanfranchi, A. Pedovani, P. Apostoli,
Free copper in serum: An analytical challenge and its possible applications. J. Trace Elem. Med. Biol., 45 (2018) 176–180.
DOI: 10.1016/j.jtemb.2017.11.006.

M.C. Linder,
Ceruloplasmin and other copper binding components of blood plasma and their functions: an update. Metallomics, 8 (2016) 887–905.
DOI: 10.1039/c6mt00103c .

A. Cabrera, E. Alonzo, E. Sauble, Y.L. Chu, D. Nguyen, M.C. Linder, D.S. Sato, A.Z. Mason,
Copper binding components of blood plasma and organs, and their responses to influx of large doses of (65)Cu, in the mouse. Biometals, 21/5 (2008) 525–543.
DOI: 10.1007/s10534-008-9139-6.
P. Deschamps, P.P. Kulkarni, M. Gautam-Basak, B. Sarkar,
The saga of copper(II)-L-histidine. Coord. Chem. Rev., 249 (2005) 895–909.
DOI: 10.1016/j.ccr.2005.09.013.

J. Masuoka, P. Saltman,
Zinc(II) and copper(II) binding to serum albumin. A comparative study of dog, bovine, and human albumin. J. Biol. Chem., 269/41 (1994) 25557–25561. available from:
https://www.jbc.org/content/269/41/25557.long
P.Z. Neumann, A. Sass-Kortsak,
The state of copper in human serum: evidence for an amino acid-bound fraction. J. Clin. Invest., 46 (1967) 646–658.
DOI: 10.1172/JCI105566 Related EVISA Resources
Related EVISA Resources
 Related EVISA News (newest first)
Related EVISA News (newest first)
last time modified: July 3, 2025