The Food and Drug Administration is investigating the risk of brain deposits after recurring use of gadolinium-based contrast agents for MRI, the agency announced in a statement.
Background:
Gadolinium-based contrast agents are used to increase the visibility of internal body structures in MRI scans. MRI contrast agents may be administered by injection or orally. While oral administration is well suited to gastrointestinal tract (GI) scans, intravascular administration proves more useful for most other scans. Today, more than 10 million GBCA injections are performed annually for purposes of medical imaging. Gadolinium, which is cytotoxic in humans, could be safely administered to patients intravenously when bonded with an organic ligand, which surrounds the gadolinium and acts as a chaperone to escort it into—and out of—the body
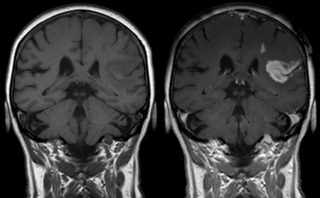
Effect of contrast agent on images: Defect of the
blood–brain barrier after stroke shown in MRI.
T1-weighted images, left image without, right image
with contrast medium administration.
|
In patient with normal kidney function GBCAs are mostly eliminated from the body through the kidneys within few hours, but trace amounts of gadolinium may stay in the body long-term, according to the FDA. Since the safety of GBCAs is partially based on the fact that their residence time in the body is very short, researchers advise that GBCAs should not be given to any individual with chronic kidney disease.
:
Recent studies suggest that gadolinium-based contrast agent (GBCA) may stay in the brains of patients, even if these have normal kidney function.
According to FDA, it is unknown whether these deposits cause adverse effects. FDA’s National Center for Toxicological Research (NCTR) will further investigate safety risks in consultation with researchers and industry, the statement said. Based on the information available at this time, the FDA is not requiring manufacturers to make changes to the labels of GBCA products. The agency is, however, recommending that clinicians limit GBCA use to situations in which the additional information provided by the contrast is necessary for patient care.
“Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols,” the FDA said.
The FDA encourages health care professionals and patients to report side effects and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program:
www.fda.gov/MedWatch/report Related information:
Related information:
 FDA: Gadolinium-based Contrast Agents for Magnetic Resonance Imaging (MRI): Drug Safety Communication - FDA Evaluating the Risk of Brain Deposits With Repeated Use
FDA: Gadolinium-based Contrast Agents for Magnetic Resonance Imaging (MRI): Drug Safety Communication - FDA Evaluating the Risk of Brain Deposits With Repeated Use Related studies:
Related studies:

Emanuel Kanal, Michael F. Tweedle,
Residual or Retained Gadolinium: Practical Implications for Radiologists and Our Patients, Radiology, 275/3 (2015) 630-634.
DOI: 10.1148/radiol.2015150805 
Robert J. McDonald, Jennifer S. McDonald, David F. Kallmes, Mark E. Jentoft, David L. Murray, Kent R. Thielen, Eric E. Williamson, Laurence J. Eckel,
Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging, Radiology, 275/3 (2015) 772-782.
doi: 10.1148/radiol.15150025

Alexander Radbruch, Lukas D. Weberling, Pascal J. Kieslich, Oliver Eidel, Sina Burth, Philipp Kickingereder, Sabine Heiland, Wolfgang Wick, Heinz-Peter Schlemmer, Martin Bendszus,
Gadolinium Retention in the Dentate Nucleus and Globus Pallidus Is Dependent on the Class of Contrast Agent, Radiology, 275/3 (2015) 783-791.
DOI: 10.1148/radiol.2015150337
Tomonori Kanda, Marie Osawa, Hiroshi Oba, Keiko Toyoda, Jun’ichi Kotoku, Takahiro Haruyama, Koji Takeshita, Shigeru Furui,
High Signal Intensity in Dentate Nucleus on Unenhanced T1-weighted MR Images: Association with Linear versus Macrocyclic Gadolinium Chelate Administration, Radiology, 275/3 (2015) 803-809.
DOI: 10.1148/radiol.14140364
Tomonori Kanda, Toshio Fukusato, Megumi Matsuda, Keiko Toyoda, Hiroshi Oba, Jun’ichi Kotoku, Takahiro Haruyama, Kazuhiro Kitajima, Shigeru Furui,
Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy, Radiology, 276/1 (2015) 228-232.
DOI: 10.1148/radiol.2015142690 Related EVISA Resources
Related EVISA Resources
 Brief summary: Speciation analysis for the study of metallodrugs and their biomolecular interactions
Brief summary: Speciation analysis for the study of metallodrugs and their biomolecular interactions  Brief summary: ICP-MS - A versatile detection system for speciation analysis
Brief summary: ICP-MS - A versatile detection system for speciation analysis  Brief summary: LC-ICP-MS - The most often used hyphenated system for speciation analysis
Brief summary: LC-ICP-MS - The most often used hyphenated system for speciation analysis  Brief summary: Chemical speciation analysis for the life sciences
Brief summary: Chemical speciation analysis for the life sciences Glossary: Metallodrugs
Glossary: Metallodrugs
 Journal Database: Journals related to Medicinal Chemistry
Journal Database: Journals related to Medicinal Chemistry Journal Database: Journals related to Pharmacology and Pharmacy
Journal Database: Journals related to Pharmacology and Pharmacy
 Journal Database: Journals related to Metallomics
Journal Database: Journals related to Metallomics
 Link Database: Research groups working on metallodrugs
Link Database: Research groups working on metallodrugs
 Directory of scientists: Researchers working on metallodrugs
Directory of scientists: Researchers working on metallodrugs Link page: All about Pharmacy and Pharmaceutical Sciences
Link page: All about Pharmacy and Pharmaceutical Sciences Related EVISA News
Related EVISA News
 March 4, 2015: Detection of Gd-based contrast agent in the skin of a patient eight years after administration
March 4, 2015: Detection of Gd-based contrast agent in the skin of a patient eight years after administration September 15, 2010: US FDA Announces Gadolinium-Based MRI Contrast Agent Warning
September 15, 2010: US FDA Announces Gadolinium-Based MRI Contrast Agent Warning March 25, 2010: Publication on the separation of Gd-based contrast agents awarded
March 25, 2010: Publication on the separation of Gd-based contrast agents awarded May 4, 2009: Gadolinium speciation analysis in search for the cause of nephrogenic systemic fibrosis (NSF)
May 4, 2009: Gadolinium speciation analysis in search for the cause of nephrogenic systemic fibrosis (NSF)  April 17, 2009: Gadolinium-based MRI contrast agents found intact in the outlet of a waste water treatment plant
April 17, 2009: Gadolinium-based MRI contrast agents found intact in the outlet of a waste water treatment plantlast time modified: November 29, 2015