A group of scientists from Münster (Germany) and Graz (Austria) have developed a method for speciation analysis for novel and established technetium radiopharmaceuticals based on liquid chromatography (LC) coupled with elemental and molecular mass spectrometry.
Background:Technetium (Tc), as the lightest element lacking stable isotopes, occurs naturally only in trace amounts. Notably, the isotope 99mTc has emerged as the predominant choice for formulating radiopharmaceuticals, being employed in over 30 million scintigraphy scans globally annually, constituting an estimated 85% of such procedures. With a half-life of approximately 6 hours, 99mTc undergoes decay into its more stable nuclear isomer, 99Tc, emitting a 140.5 keV gamma quantum. This emission is crucial for single-photon emission computed tomography (SPECT), a technique utilized with scintillation cameras in diagnostic imaging. Leveraging the broad redox chemistry of Tc enables the synthesis of complexes tailored for specific endogenous structures and organs.
To obtain 99mTc, generators containing 99Mo as the mother nuclide are utilized. Pertechnetate is obtained on-site through chromatography and processed into desired tracers using kit formulations. The short half-life necessitates swift formulation and timely scintigraphy, which can pose challenges for quality controls and contrast generation. Unfortunately, the low concentrations used in scintigraphy also limit routine analytical techniques, hindering both quality controls and the development of novel tracers.
The new study:In a recent study, scientists from Münster (Germany) and Graz (Austria) introduced a groundbreaking method for speciation analysis of both new and established Tc radiopharmaceuticals. This innovative approach involves liquid chromatography (LC) coupled with elemental and molecular mass spectrometry, enabling direct identification, characterization, and quantification (refer to the figure below).
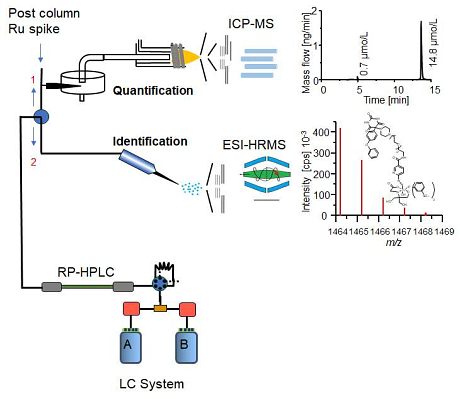
The researchers employed an Orbitrap-based ESI-MS method to achieve high mass resolutions and accuracies for compound identification. Pertechnetate, identified as an impurity in all formulations, was precisely characterized through high mass accuracies and retention time comparisons. Additionally, unknown impurities were detected for some tracers using RP-HPLC-ICP-MS, a method that allows for the separation and sensitive detection of Tc species. Due to the absence of Tc standards, the previously introduced "
isobaric dilution analysis" was modified and implemented in an on-line post-column dilution approach. This comprehensive technique not only identified and quantified various Tc impurities in a non-target approach but also facilitates rapid on-site quality controls for commercially available tracers. Moreover, it eliminates the need for "cold" reference compounds, thereby advancing the characterization of experimental tracers in nuclear medicine.
 Related Studies:
Related Studies:
 D. Clases,
D. Clases, R. Gonzalez de Vega,
Facets of ICP-MS and their potential in the medical sciences—Part 1: fundamentals, stand-alone and hyphenated techniques, Anal. Bioanal. Chem., 414 (2022) 7337–7361.
DOI: 10.1007/s00216-022-04259-1
 D. Clases
D. Clases,
M. Sperling,
U. Karst,
Analysis of metal-based contrast agents in medicine and the environment, Trends Anal. Chem., 104 (2018) 135–147.
DOI: 10.1016/j.trac.2017.12.011 D. Clases
D. Clases, M. Birka,
M. Sperling, A. Faust,
U. Karst,
Isobaric dilution analysis as a calibration tool for long lived radionuclides in ICP-MS, J. Trace Elem. Med. Biol., 40 (2017) 97–103.
DOI: 10.1016/j.jtemb.2017.01.002
M.U. Akbar, M.R. Ahmad, A. Shaheen, S. Mushtaq,
A review on evaluation of technetium-99m labeled radiopharmaceuticals, J. Radioanal. Nucl. Chem., 310/2 (2016) 477–493.
DOI: 10.1007/s10967-016-5019-7
F.E. Buroni, L. Lodola, M.G. Persico, C. Aprile,
Deficiencies in product labelling instructions and quality control directions for 99mTc radiopharmaceuticals, Nucl. Med. Commun., 35/2 (2014) 197–204.
DOI: 10.1097/MNM.0000000000000030
T.C. Pinkerton, W.R. Heineman, E. Deutsch,
Separation of technetium hydroxyethylidene diphosphonate complexes by anion-exchange high performance liquid chromatography, Anal. Chem., 52/7 (1980) 1106–1110.
DOI: 10.1021/ac50057a025