Researchers from Germany and France have developed a strategy to obtain information about gadolinium species that degrade during extraction from bone tissue.
Background:Gadolinium-based contrast agents (GBCAs) are intravenous drugs used in diagnostic imaging procedures to enhance the quality of magnetic resonance imaging. Safety claims for the GBCAs are based on the high stability of the agents and the high solubility, leading to rapid excretion in an intact state. Anyhow, since 2016 it has been established that small fractions of the GBCAs are retained for extended times within brain, bone and other organs. In order to better understand the mechanism of retention, the information on the structure of the retained gadolinium (Gd) species is of greatest importance. Unfortunately, the concentration of the retained species is too small for getting direct speciation information via X-ray absorption spectroscopy from tissue samples. The most often used speciation technique for GBCAs, namely HPLC-ICP-MS, is based on the analysis of solutions and therefore calls for the extraction of the retained species. Extraction of strongly bound species from tissue samples is challenging, since mild extraction conditions using aqueous solutions cannot extract significantly fractions of the retained species and harsher conditions might destroy the species retained. Such dilemma is especially cumbersome for bone tissues, calling for some solubilization before extraction can be envisaged.
The new study: A team of researchers from Germany and France now propose a strategy to circumvent the loss of species information during extraction of solid sample materials. The general procedure of the new method is outlined in Figure 1. The tested GBCAs were Dotarem (Gd-DOTA) (Guerbet), Omniscan (Gd-DTPABMA) (GE Healthcare), MultiHance (Gd-BOPTA) (Bracco), Gadovist (Gd-BT-DO3A) (Bayer), and ProHance (Gd-HPDO3A) (Bracco). The developed method was carried out on rats which received one of the tested GBBCAs one month before being sacrificed.
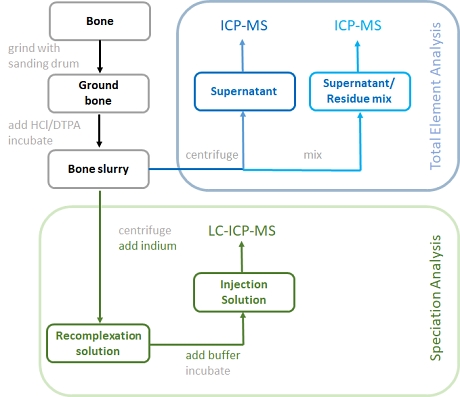
Figure 1: Schematic representation of the sample pretreatment for bone analysis
The sample preparation starts by creating a fine powder from the bone sample. This step was essential in order to support a rapid dissolution of the bone material with relatively low concentration of hydrochloric acid. In order to complex any free Gd3+, DTPA was added to the acid for digestion.
The Gd content within the dissolved bone sample (bone slurry) was determined by ICP-MS after centrifugation. The analysis of the supernatant gave the "Total dissolved Gd" while the analysis of the mixed residue/supernatant was done in order to measure the extraction efficiency. The gadolinium was quantitatively recovered in the supernatant either as intact GBCA, or as Gd-DTPA complex.
For Gd speciation analysis, the bone slurry was first centrifuged and the supernatant was mixed with an Indium solution. The addition of indium was meant to capture any chelate resulting from the release of Gd from the original Gd-complex.
After incubation of the solution within an ultrasonic bath for 30 min at 40 °C, buffer was added to create the final injection solution for HPLC-ICP-MS analysis. Chromatographic separation was based on the use of a HILIC column eluted under isocratic conditions with 65% ACN an d 35% 50 mM ammonium formate at pH 5.5.
The evaluation of the analytical results revealed that the macrocyclic GBCAs Gd-DOTA, Gd-HPDO3A and Gd-Gd-BT-DO3A survived the bone dissolution step, while the linear GBCAs dissociated due to their lower stability. Linear, unstable GBCAs dechelate into gadolinium and their respective ligand. The gadolinium released from its complex during the acid treatment is re-complexed by DTPA added to the bone dissolution acid. The gadolinium which was present in the bone in free form is also complexed by DTPA.
In order to recover some species information lost during the bone dissolution step, indium was added in large excess. This led to a recomplexation of the released GBCA ligands with indium, while keeping the intact GBCAs unchanged.
Rats treated with linear GBCAs showed the highest amounts of total retained Gd with a maximum of 171 nmol/g for Gd-DTPA-BMA. Total Gd concentration in bones of rats treated with macrocyclic GBCAs were at least one order of magnitude lower. The results also confirm that the Gd retained in bone of mice treated with linear GBCAs is mostly present as dechelated from its original complex.
The same method was applied to bone marrow samples of the same animals. Although all macrocyclic GBCAs behaved similarly in both matrices, linear GBCAs showed a much higher gadolinium accumulation in bone, indicating a different biodistribution behavior.
 The original publication
The original publication
Lukas Schlatt, Alexander Köhrer, Cécile Factor, Philippe Robert, Marlène Rasschaert,
Michael Sperling, and
Uwe Karst,
Mild Dissolution/Recomplexation Strategy for Speciation Analysis of Gadolinium from MR Contrast Agents in Bone Tissues by Means of HPLC-ICP-MS, Anal. Chem., 93/33 (2021) 11398−11405.
DOI: 10.1021/acs.analchem.1c01100
 Instrumentation used:
Instrumentation used:
 Thermo Scientific - iCAP-TQ ICP-MS
Thermo Scientific - iCAP-TQ ICP-MS Thermo Scientific - Dionex UltiMate 3000 HPLC
Thermo Scientific - Dionex UltiMate 3000 HPLC Related studies (newest first):
Related studies (newest first):

S. Bussi, A. Coppo, R. Celeste, A. Fanizzi, A. Fringuello Mingo, A. Ferraris, C. Botteron, M.A. Kirchin, F. Tedoldi, F. Maisano,
Macrocyclic MR contrast agents: evaluation of multiple-organ gadolinium retention in healthy rats, Insights Imaging 2020, 11, No. 11.
DOI: 10.1186/s13244-019-0824-5

Mariane Le Fur, Peter Caravan,
The biological fate of gadolinium-based MRI contrast agents: a call to action for bioinorganic chemists, Metallomics, 11(2 (2019) 240-254.
DOI: 10.1039/c8mt00302e
N. Fretellier, A. Granottier, M. Rasschaert, A.-L. Grindel, F. Baudimont, P. Robert, J.-M. Idée, C. Corot,
Does Age Interfere With Gadolinium Toxicity and Presence in Brain and Bone Tissues? A Comparative Gadoterate Versus Gadodiamide Study in Juvenile and Adult Rats, Invest. Radiol., 54 (2019) 61−71.
DOI: 10.1097/RLI.0000000000000517
E. Di Gregorio, R. Iani, G. Ferrauto, R. Nuzzi, S. Aime, E.J. Gianolio,
Gd accumulation in tissues of healthy mice upon repeated administrations of Gadodiamide and Gadoteridol, Trace Elem. Med. Biol., 48 (2018) 239−245.
DOI: 10.1016/j.jtemb.2018.04.018
S. Bussi, A. Coppo, C. Botteron, V. Fraimbault, A. Fanizzi, E. De Laurentiis, S. Colombo Serra, M.A. Kirchin, F. Tedoldi, F.J. Maisano,
Differences in Gadolinium Retention After Repeated Injections of Macrocyclic MR Contrast Agents to Rats, Magn. Reson. Imaging, 47 (2018) 746−752.
DOI: 10.1002/jmri.25822

M. Rasschaert, J.-M. Idée, P. Robert, N. Fretellier, V. Vives, X. Violas, S. Ballet, C. Corot,
Moderate Renal Failure Accentuates T1 Signal Enhancement in the Deep Cerebellar Nuclei of Gadodiamide-Treated Rats, Invest. Radiol., 52 (2017) 255−264.
DOI: 10.1097/RLI.0000000000000339
E. Lancelot,
Revisiting the Pharmacokinetic Profiles of Gadolinium-Based Contrast Agents - Differences in Long-Term Biodistribution and Excretion, Invest. Radiol., 51 (2016) 691−700.
DOI: 10.1097/RLI.0000000000000280 
A.A.P. Kartamihardja, T. Nakajima, S. Kameo, H. Koyama, Y. Tsushima,
Impact of Impaired Renal Function on Gadolinium Retention After Administration of Gadolinium-Based Contrast Agents in a Mouse Model, Invest. Radiol. 2016, 51, 655−660.
DOI: 10.1097/RLI.0000000000000295
N. Murata, L.F. Gonzalez-Cuyar, K. Murata, C. Fligner, R. Dills, D. Hippe, K.R. Maravilla,
Macrocyclic and Other Non–Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue Preliminary Results From 9 Patients With Normal Renal Function, Invest. Radiol. 2016, 51, 447−453.
DOI: 10.1097/RLI.0000000000000252
N. Murata, K. Murata, L.F. Gonzalez-Cuyar, K.R. Maravilla,
Gadolinium tissue deposition in brain and bone, Magn. Reson. Imaging, 34 (2016) 1359−1365.
DOI: 10.1016/j.mri.2016.08.025
Y.-X.J. Wáng, J. Schroeder, H. Siegmund, J. Idée, N. Fretellier, G. Jestin-Mayer, C. Factor, M. Deng, W. Kang, S.K. Morcos,
Total gadolinium tissue deposition and skin structural findings following the administration of structurally different gadolinium chelates in healthy and ovariectomized female rats, Quant. Imaging Med. Surg., 5 (2015) 534−545.
DOI: 10.3978/j.issn.2223-4292.2015.05.03
C. Vidaud, D. Bourgeois, D. Meyer,
Bone as Target Organ for Metals: The Case of f-Elements, Chem. Res. Toxicol., 25 (2012) 1161−1175.
DOI: 10.1021/tx300064m
N. Fretellier, J.-M. Idée, A. Dencausse, O. Karroum, S. Guerret, N. Poveda, G. Jestin, C. Factor, I. Raynal, P. Zamia, M. Port, C. Corot,
Comparative In Vivo Dissociation of Gadolinium Chelates in Renally Impaired Rats, A Relaxometry Study, Invest. Radiol., 46 (2011) 292−300.
DOI: 10.1097/RLI.0b013e3182056ccf
T.H. Darrah, J.J. Prutsman-Pfeiffer, R.J. Poreda, M.E. Campbell, P.V. Hauschka, R.E. Hannigan,
Incorporation of excess gadolinium into human bone from medical contrast agents, Metallomics, 1 (2009) 479−488.
DOI: 10.1039/b905145g
S. Bussi, X. Fouillet, A. Morisetti,
Toxicological assessment of gadolinium release from contrast media, Exp. Toxicol. Pathol., 58 (2007) 323−330.
DOI: 10.1016/j.etp.2006.09.003
G.W. White, W.A. Gibby, M.F. Tweedle,
Comparison of Gd(DTPA-BMA) (Omniscan) Versus Gd(HP-DO3A) (ProHance) Relative to Gadolinium Retention in Human Bone Tissue by Inductively Coupled Plasma Mass Spectroscopy, Invest. Radiol., 41 (2006) 272−278.
DOI: 10.1097/01.rli.0000186569.32408.95
W.A. Gibby, K.A. Gibby, W.A. Gibby,
Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) Retention in Human Bone Tissue by Inductively Coupled Plasma Atomic Emission Spectroscopy, Invest. Radiol., 39 (2004) 138−142.
DOI: 10.1097/01.rli.0000112789.57341.01
 Related EVISA News
Related EVISA News
last time modified: September 19, 2024