A group of Chinese researchers now studied the transformation kinetics of different arsenic species in aqueous solution by ultrasonic treatment in order to select optimum conditions for efficient extraction with minimum species transformation.
Background:High performance liquid chromatography-inductively coupled plasma-mass spectrometry (HPLC-ICP-MS) is the most often used technique for speciation analysis. The application of this technique for speciation analysis is relatively straightforward when the sample is an aqueous solution, such as water or urine. The situation becomes more complicated, when solid samples have to be analysed. For accurate determination of different species, not only is a quantitative extraction required, but also species transformation during extraction has to be avoided. Since oxidative destruction of the sample matrix cannot be applied because analyte speciation cannot be maintained during such sample treatment, only relatively mild extraction procedures can be used. An often used technique for such task is ultrasonic assisted extraction. While the ultrasonic treatment is enhancing the extraction efficiency, the question is whether the different species will be transformed during such energetic treatment.
The new study:A group of Chinese researchers now studied the transformation kinetics of different arsenic species in aqueous solution by ultrasonic treatment. The used conditions were meant to mimic ultrasound-assisted extraction of these species from solid samples such as animal tissues, plant materials or food samples. Considering the complexity of sample matrix, too low ultrasonic powers or too high ultrasonic water bath temperatures will make the cavitation effect weak, thus resulting in the poor extraction efficiency. Therefore, the researchers used relatively high ultrasonic power of 300 W and investigated the transformation kinetics of arsenic species at different ultrasonic water bath temperatures.
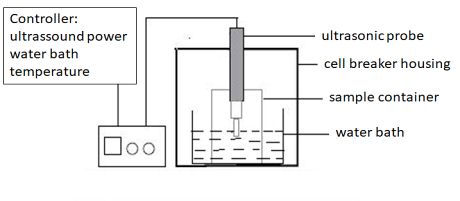
Figure: Experimental setup
Another set of experiments were performed at relatively low water bath temperature of 60 °C and investigating the transformation kinetics of arsenic species at different ultrasonic power settings. The species investigated were arsenite (As(III)), arsenocholine (AsC) and arsenobetaine (AsB). These species were identified as the least stable species in prior experiments.
Inorganic arsenic (As(III))
When using 300 W ultrasonic power and fixed water bath temperature, the conversion rate of As(III) increased with the treatment time. When the treatment time was fixed, the conversion rate of As(III) was significantly higher for a bath temperature of 20°C in comparison to 0°C. For higher than 20°C bath temperatures, the conversion rates decreased with increasing bath temperatures. At 20°C, near to 96% conversion was achieved within 60 min. This is somehow astonishing, since the oxidation rate of As(III) in pure oxygen is rather slow. The results obtained here clearly show that ultrasonic treatment can significantly promote the transformation of As(III) to As(V). When using a water bath temperature of 60°C and fixed treatment time, the conversion rate of As(III) increased with increasing ultrasonic power up to 600 W. The kinetics of the reaction were further investigated by fitting 1st- and 2nd-order kinetic models to the data. A better correlation coefficient was obtained for first-order kinetics, indicating that this model is more consistent with the observed rate curve. The results also showed that the transformation reaction was endothermic, and the activation energy was positive.
Arsenocholine/arsenobetaine
When the ultrasonic power was set to 300W and bath temperature was 20 °C, the conversion of arsenocholine reached 40.33 % while conversion of arsenobetaine only 15.89%. The conversion rate for both compounds declined with increasing temperatures above 20°C. Using a water bath temperature of 60°C and a fixed treatment time, the conversion rate increased with increasing ultrasonic power up to 600 W. Again, the kinetics could be well described by a first-order model.
The experiments clearly show that the observed relations are the result of the synergy between thermal effects and ultrasonic cavitation effect. When the liquid temperature is too high, the vapour pressure in the bubbles increases, so that the buffering effect is enhanced and the ultrasonic cavitation is reduced when the bubbles close, thus reducing the transformation efficiency. When the ultrasonic intensity increases, the cavitation bubble collapses violently, which enhances the cavitation effect and has positive effects on the transformation efficiency. However, when the cavitation effect tends to be saturated, increasing the ultrasonic intensity will generate numerous useless bubbles, which enhances the scattering attenuation and weakens the cavitation effect, thus inhibiting the transformation reaction.
The authors conclude that ultrasonic treatment conditions with stable arsenic species (150–300 W, 40-60 °C, 5–10 min) should be selected to optimize the ultrasound-assisted extraction method of arsenic species in edible fungi, and then get the ultrasound-assisted extraction conditions with relatively low transformation rate of arsenic species and relatively high extraction rate of arsenic species in edible fungi.
 The original study:
The original study:

Yifan Wang, Shuangyang Chen, Donglu Fang, Chunli Song, Liyan Zhao,
Ultrasonic treatment of arsenic species: Transformation kinetics analysis, Microchemical Journal 157 (2020) 105068.
DOI: 10.1016/j.microc.2020.105068
 Used techniques and instrumentation:
Used techniques and instrumentation:
 Related studies (newest first)
Related studies (newest first)

S.Y. Chen, B.M. Kimatu, D. Fang, X. Chen, G. Chen, Q. Hu, L. Zhao,
Effect of ultrasonic treatment on transformations of arsenic species in edible mushrooms, Anal. Lett. 53 (2020) 102–121.
DOI: 10.1080/00032719.2019.1639056
R.C. Assis, B.A. de Araújo Faria, C.L. Caldeira, A.B. Mageste, L.R. de Lemos, G.D. Rodrigues,
Extraction of arsenic(III) in aqueous two-phase systems: a new methodology for determination and speciation analysis of inorganic arsenic, Microchem. J. 147 (2019) 429–436.
DOI: 10.1016/j.microc.2019.03.058

F. Chemat, N. Rombaut, A.-G. Sicaire, A. Meullemiestre, A.-S. Fabiano-Tixier, M. Abert-Vian,
Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review, Ultrason. Sonochem. 34 (2017) 540–560. DOI:
10.1016/j.ultsonch.2016.06.035

S. Phongsirirux, P. Sricharoen, N. Limchoowong,
Mild acid ultrasonic assisted extraction of arsenic residues in different parts of hot chilli prior to ultra-trace determination by flow injection-hydride generation atomic absorption spectrometry, Orient. J. Chem. 33 (2017) 2347–2355.
DOI: 10.13005/ojc/330526

M. D'Amato, F. Aureli, S. Ciardullo, A. Raggi, F. Cubadda,
Arsenic speciation in wheat and wheat products using ultrasound-and microwave-assisted extraction and anion exchange chromatography-inductively coupled plasma mass spectrometry, J. Anal. At. Spectrom. 26 (2011) 207–213.
DOI: 10.1039/c0ja00125b

A. Väisänen, R. Suontamo, J. Silvonen, J. Rintala,
Ultrasound-assisted extraction in the determination of arsenic, cadmium, copper, lead, and silver in contaminated soil samples by inductively coupled plasma atomic emission spectrometry, Anal. Bioanal. Chem. 373 (2002) 93–97.
DOI: 10.1007/s00216-002-1290-2

V. Devesa, A. Martinez, M.A. Suner, V. Benito, D. Velez, R. Montoro,
Kinetic study of transformations of arsenic species during heat treatment, J. Agric. Food. Chem. 49 (2001) 2267–2271.
DOI: 10.1021/jf001328e
 Related EVISA News
Related EVISA News