A new HPLC column has been developed by Agilent that allows for the routine determination of all five major arsenic species in human urine in a single HPLC run taking less than 12 minutes.
Background:While arsenic is often considered to be the synonym for toxin, arsenic toxicity depends strongly on the species being present. Humans are exposed primarily through ingestion via diet (drinking water, seafood) or inhalation and workplace exposure. Arsenic is used in the manufacture of glass, pigments, medicinals, pesticides, wood preservation and semiconductor products. Once ingested, arsenic gets metabolised to a certain degree depending on speciation end level of exposure and is predominantly excreted in the urine. Therefore urinary measurement is an excellent biomarker for As speciation and differentiation of arsenic species is valuable information to trace the route of exposure. However various obstacles have prevented the speciation analysis of arsenic in urine from becoming routine:
- finding chromatographic conditions that allow the determination of the main As species in one run without changing columns (anionic, cationic, etc.)
- achieving good reproducible separation with high sensitivity and dynamic range without complicated gradient conditions
- resolving or eliminatimng the ArCl interference on As which is derived from the high NaCl concentration present in urine
- aciving long time stability despite the high total dissolved solids (TDS) resulting from the sample matrix and HPLC buffer systems.
The new column material:Since none of the various commercially available columns proved 100% satisfactory at meeting the Agilent application goals, a new column was developed and manufactured (Agilent G3288-8000, 4.6 x 250 mm plus G3154-65002 Guard column). The new column provides excellent resolution of As(III) from both arsenobetaine and DMA, as well as good separation of MMA from chloride. Together with the guard column, this material allows for the analysis of indiluted 5-µL aliquots of human urine samples.
Mobile phase:In order to buffer the changing matrix between different urine samples but also avoid excessive total salt concentrations that would compromise long term stability, a mixture of sodium acetate and sodium nitrate was used as the mobile phase. For enhancing the sensitivity, 1% of ethanol was added as a modifier.
Results:
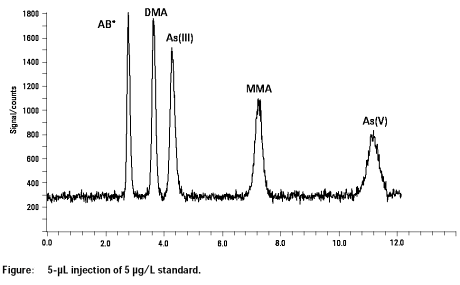
All five main arsenic species present in human urine (As(III), As(V), AB, MMNA, DMA) were resolved from each other and from chloride within a separation time of 12 min. Detection limits were excellent and ranged from 0.05 to 0.1 µg/l depending on species. Reproducibility of peak area was better than 3% at 10 µg/L and reproducibility of the retention time was better than 0.6%. The arsenic species determined in the CRM NIES No. 18 agreeed well with the certified values vor DMA and AB.
The full report can be downloaded from Agilent's server as an application note:
 Tetsushi Sakai, Steve Wilbur, Routine Analysis of Toxic Arsenic Species in Urine Using HPLC with ICP-MS, Agilent Application Note, August 25, 2006, 5989-5505EN
Tetsushi Sakai, Steve Wilbur, Routine Analysis of Toxic Arsenic Species in Urine Using HPLC with ICP-MS, Agilent Application Note, August 25, 2006, 5989-5505EN Related studies:
Related studies: Marie E. Vahter
Marie E. Vahter,
What Are the Chemical Forms of Arsenic in Urine, and What Can They Tell Us About Exposure ?, Clin. Chem. (Winston-Salem, N.C.), 40/5 (1994) 679-680.

J. Lintschinger, P. Schramel, A. Hatalak-Rauscher, I. Wendler,
B. Michalke,
A new method for the analysis of arsenic species in urine by using HPLC-ICP MS, Fresenius J. Anal. Chem., 362/3 (1998) 313-318.
doi: 10.1007/s002160051080 R. Ritsema
R. Ritsema, L. Dukan, T.R. i Navarro, W. van Leeuwen, N. Oliveira, P. Wolfs, E. Lebret,
Speciation of arsenic compounds in urine by LC-ICP MS, Appl. Organomet. Chem., 12/8-9 (1998) 591-599.
DOI: 10.1002/(SICI)1099-0739(199808/09)12%3A8/9<591%3A%3AAID-AOC767>3.0.CO%3B2-E
J. Zheng,
W. Goessler, W. Kosmus,
Speciation of arsenic compounds by coupling high-performance liquid chromatography with inductively coupled plasma mass spectrometry, Mikrochim. Acta (Wien), 130/1-2 (1998) 71-79.
doi: 10.1007/BF01254593 Mariella Moldovan
Mariella Moldovan,
M. Milagros Gómez, M. Antonia Palacios,
Carmen Cámara,
Arsenic speciation in water and human urine by HPLC-ICP-MS and HPLC-MO-HG-AAS, Microchem. J., 59/1 (1998) 89-99.
doi: 10.1006/mchj.1997.1556 Jörg Feldmann
Jörg Feldmann, V.W.-M. Lai, W.R. Cullen, M. Ma, X. Lu, X.C. Le,
Sample preparation and storage can change arsenic speciation in human urine, Clin. Chem. (Winston-Salem, N.C.), 45 (1999) 188-1997. Full text free available at:
http://intl.clinchem.org/cgi/content/abstract/45/11/1988
Gautam Samanta, Uttam K. Chowdhury, Badal K. Mandal, Dipankar Chakraborti, N. Chandra Sekaran, Hiroshi Tokunaga, Masanori Ando,
High performance liquid chromatography inductively coupled plasma mass spectrometry for speciation of arsenic compounds in urine, Microchem. J., 65/2 (2000) 113-127.
doi: 10.1016/S0026-265X(00)00039-4
Tetsushi Sakai, Yoshinori Inoue, Yukiko Date, Tetsuya Aoyama, Kaoru Yoshida, Ginji Endo,
Simultaneous determination of neutral, anionic and cationic compounds within one chromatographic run using an inductively coupled plasma mass spectrometer as element-specific detector, Appl. Organomet. Chem., 15/4 (2001) 285-290.
doi: 10.1002/aoc.141
K. Wróbel, K. Wróbel, B. Parker,
S.S. Kannamkumarath,
Joseph A. Caruso,
Determination of As(III), As(V), monomethylarsonic acid, dimethylarsinic acid and arsenobetaine by HPLC-ICP-MS: analysis of reference materials, fish tissues and urine, Talanta, 58/5 (2002) 899-907.
doi: 10.1016/S0039-9140(02)00404-6
Yen-Ching Chen, Chitra J. Amarasiriwardena, Yu-Mei Hsueh, David C. Christiani,
Stability of Arsenic Species and Insoluble Arsenic in Human Urine, Cancer Epidemiol. Biomarkers Prev., 11 (2002) 1427-1433. Full text available at:
http://cebp.aacrjournals.org/cgi/content/abstract/11/11/1427
Lisa S. Milstein, Amal Essader, Edo D. Pellizzari, Reshan A. Fernando, Jame
H. Raymer, Keith E. Levine, Olujide Akinbo, Development and Application of a Robust
Speciation Method for Determination of Six Arsenic Compounds Present in Human Urine, Environ. Health Perspect., 111/3 (2003) 293-296.
http://ehp.niehs.nih.gov/docs/2003/5525/abstract.html Jens J. Sloth
Jens J. Sloth,
Erik H. Larsen, Kĺre Julshamn,
Selective arsenic speciation analysis of human urine reference materials using gradient elution ion-exchange HPLC-ICP-MS, J. Anal. At. Spectrom., 19/8 (2004) 973-978.
doi: 10.1039/b402994a
Vivian W.M. Lai, Yongmei Sun, Eon Ting, William R. Cullen, Kenneth J. Reimer,
Arsenic speciation in human urine: are we all the same ?, Toxicol. Appl. Pharmacol., 198/3 (2004) 297-306.
doi: 10.1016/J.TAAP.2003.10.033

Todor I. Todorov, John W. Ejnik, Florabel G. Mullick, Jose A. Centeno,
Arsenic Speciation in Urine and Blood Reference Materials, Mikrochim. Acta (Wien), 151/3-4 (2005) 263-268.
doi: 10.1007/s00604-005-0414-8
S. García Salgado, M.A. Quijano Nieto, M.M. Bonilla Simón,
Determination of soluble toxic arsenic species in alga samples by microwave-assisted extraction and high performance liquid chromatography-hydride generation-inductively coupled plasma-atomic emission spectrometry, J. Chromatogr. A, 1129/1 (2006) 54-60.
doi:10.1016/j.chroma.2006.06.083
Ruimin Xie, Willie Johnson, Steve Spayd, Gene S. Hall, Brian Buckley,
Arsenic speciation analysis of human urine using ion exchange chromatography coupled to inductively coupled plasma mass spectrometry, Anal. Chim. Acta, 578/2 (2006) 186-194.
doi:10.1016/j.aca.2006.06.076
 Related EVISA News (newest first):
Related EVISA News (newest first):
 January 10, 2023: Online addition of an internal standard for high performance liquid chromatography-inductively coupled plasma mass spectrometry
January 10, 2023: Online addition of an internal standard for high performance liquid chromatography-inductively coupled plasma mass spectrometrylast time modified: March 8, 2024