Researchers from the University of Münster have developed a new calibration strategy for quantitative bioimaging using laser ablation - inductively coupled plasma mass spectrometry based on on-line isotope dilution analysis.
Background:
Laser ablation is a well established technique for sampling solid
materials with spatial resolution. Coupled with an inductively coupled plasma source for inorganic mass spectrometry
(ICP-MS), this technique can be used for the spatially resolved
determination of trace elements. More recently, LA-ICP-MS has been used
very successfully for the imaging of trace metals in tissue sections
providing even quantitative information. The most often used quantification strategy is based on external calibration using matrix-matched standards.
This approach requires the use of standard materials for calibration that are similar in the composition with the samples to be analysed with respect to their behaviour during laser sampling (ablation behaviour) as well as with respect to their response from the ionization source (plasma behaviour). Since certified reference materials (CRMs) with sufficient homogeneous distribution of trace elements that match the composition of the biological sample materials to be analysed are seldom available, calibration standards have to be prepared by spiking appropriate materials. Since the correctness of quantification relies on the quality of such matrix-matching between sample and standards, the choice of standard material and the preparation of the standard is of utmost importance.
Bioimaging by LA-ICP-MS requires the quantification of all analytes in all pixels of the "image". Depending on the frequency of the laser sampling process, image generation is a rather time- consuming process. Therefore, for stable imaging conditions any instrumental drift should be avoided or corrected for.
The new quantification strategy:
The quantification strategy developed by the researchers from the University of Münster (Germany) is based on the Isotope Dilution Analysis (IDA) approach. IDA is a definitive quantification method, which is generally traceable to SI units. The developed method was tested and applied to the determination of Pt in tissue sections of rats treated with Cisplatin.
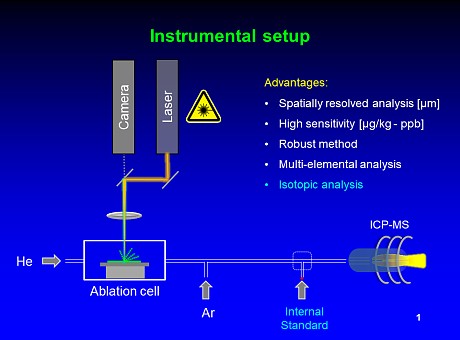
Figure 1: Instrumental setup for
on-line IDA for LA-ICP-MS: An isotope spike is added as a dry aerosol to the aerosol generated by laser ablation
A dry 194Pt spike aerosol is added in a post-cell setup (see Fig. 1), and the natural 194Pt/195Pt isotope ratio of the sample aerosol from laser ablation was changed accordingly. Prior to analysis, mass bias correction and spike characterization were performed. The 194Pt spike concentrations were determined via reversed IDA using a conventional platinum ICP standard.
The second step of the IDA workflow (see Fig. 2), the determination of the spike mass flow MFsp is the most critical point of the on-line ID-LA-ICP-MS method because the platinum mass flow of the nebulized aerosol reaching the ICP-MS has to be quantified.
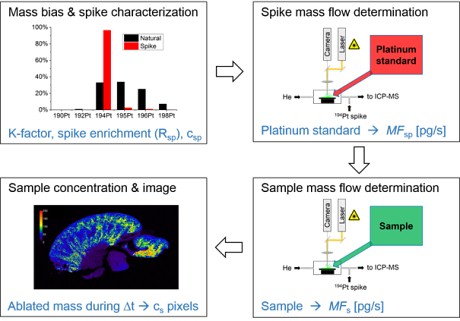
Figure 2: Conceptional approach of the
on-line ID-LA-ICP-MSA method.
Four steps are performed including mass bias and spike characterization, spike mass flow determination via reversed IDA, sample mass flow determination and finally the data processing to the respective concentrations and image generation.
(Reprinted with permission from O.B. Bauer et al., Quantitative Bioimaging of Platinum via on-line Isotope
Dilution-Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry
(ID-LA-ICP-MS), Anal. Chem., (2018). Copyright 2018 American Chemical Society)For this purpose, reversed IDA through ablation of a reference platinum standard of e.g. 100 µg/g and simultaneous 194Pt spike introduction (e.g., 125 ng/L) is performed and MFsp usually in the pg/s range. These measurements are with an inherent uncertainty, because the concentration of the platinum standard has to be determined previously, and also the ablated mass mst is calculated assuming the density and the height of the ablated slices. In the third step, the sample mass flow MFs is recorded. In order to do so, the platinum standard within the ablation chamber was exchanged with the rat kidney sample and all conditions as the nebulized spike and the LA-ICP-MS parameters were kept the same.
In the last step of the IDA approach, the respective platinum concentrations for each pixel of the resulting quantitative platinum distribution image are calculated by integrating the sample mass flow MFs for each measurement time interval Δt (the total duty cycle of the ICP) and then divided by the total mass of ablated material.
The authors could prove, that the prerequisite for IDA, namely complete mixing of sample and spike, is occurring in the aerosol on its way to the ICP. Quantitative data obtained by the new approach correlated well with those obtained by external calibration when analysing parallel tissue slices of rat kidney from Cisplatin perfusion studies. The novel quantification approach based on the definitive IDA method is traceable to SI units and can compensate signal drifts during measurements as the second isotope acts as an internal standard.
Michael Sperling
 The original studies
The original studies

Oliver Bolle Bauer, Christina Köppen,
Michael Sperling, Hans-Joachim Schurek, Giuliano Ciarimboli, and
Uwe Karst.
Quantitative Bioimaging of Platinum via on-line Isotope Dilution-Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry (ID-LA-ICP-MS), Anal. Chem., 90/11 (2018) 7033-7039. DOI: 10.1021/acs.analchem.8b01429.
 Used instrumentation:
Used instrumentation:
 CETAC: LSX-213 Laser ablation
CETAC: LSX-213 Laser ablation Agilent 7500 CE ICP-MS
Agilent 7500 CE ICP-MS
ESI APEX Q Sample introduction system
 Related studies
Related studies

I. Moraleja, M.L. Mena, A. Lázaro, B. Neumann, A. Tejedor,
N. Jakubowski, M.M. Gómez-Gómez, D. Esteban-Fernández,
An Approach for Quantification of Platinum Distribution in Tissues by LA-ICP-MS Imaging Using Isotope Dilution Analysis. Talanta, 178 (2018) 166–171.
DOI: 10.1016/j.talanta.2017.09.031

L. Feng, J. Wang, H. Li, X. Luo, J. Li,
A Novel Absolute Quantitative Imaging Strategy of Iron, Copper and Zinc in Brain Tissues by Isotope Dilution Laser Ablation ICP-MS. Anal. Chim. Acta, 984 (2017) 66–75.
DOI: 10.1016/J.aca.2017.07.003

D.N. Douglas, J. O’Reilly, C. O’Connor,
B.L. Sharp,
H. Goenaga-Infante,
Quantitation of the Fe Spatial Distribution in Biological Tissue by Online Double Isotope Dilution Analysis with LA-ICP-MS: A Strategy for Estimating Measurement Uncertainty. J. Anal. At. Spectrom., 31 (2016) 270–279.
DOI: 10.1039/c5ja00351b

Natalia Miliszkiewicz, Stanisław Walas, Anna Tobiasz,
Current approaches to calibration of LA-ICP-MS analysis, J. Anal. At. Spectrom., 30 (2015) 327-338.
DOI: 10.1039/c4ja00325j

B. Fernández, P. Rodríguez-González,
J.I. García Alonso, J. Malherbe, S. García-Fonseca, R. Pereiro,
A. Sanz-Medel,
On-Line Double Isotope Dilution Laser Ablation Inductively Coupled Plasma Mass Spectrometry for the Quantitative Analysis of Solid Materials. Anal. Chim. Acta, 851 (2014) 64–71.
DOI: 10.1016/j.aca.2014.08.017

A. Susulini, E. Wiener, T. Marnitz, B. Wu, B. Müller, B. Hamm,
J.S. Becker, Quantitative Imaging of the Tissue Contrast Agent [Gd(DTPA)]2− in Articular Cartilage by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Contrast Media Mol. Imaging, 8 (2013) 204–209.
DOI: 10.1002/cmmi.1509

Fanny Claverie, Julien Malherbe, Naomi Bier, John L. Molloy, Stephen E. Long,
Putting a spin on LA-ICP-MS analysis combined to isotope dilution, Anal. Bioanal. Chem., 405 (2013) 2289–2299.
DOI: 10.1007/s00216-012-6645-8

D. Hare, C. Austin, P. Doble,
Quantification
strategies for elemental imaging of biological samples using laser
ablation-inductively coupled plasma-mass spectrometry, Analyst, 137 (2012) 1527-1537.
DOI: 10.1039/C2AN15792F

D.J. Kutscher, M.B. Fricker, B. Hattendorf,
J. Bettmer, D. Günther, Systematic Studies on the Determination of Hg-Labelled Proteins Using Laser Ablation-ICPMS and Isotope Dilution Analysis. Anal. Bioanal. Chem., 401 (2011) 2691–2698.
DOI: 10.1007/s00216-011-5199-5

I. Konz, B. Fernández, M.L. Fernández, R. Pereiro,
A. Sanz-Medel,
Absolute Quantification of Human Serum Transferrin by Species-Specific Isotope Dilution Laser Ablation ICP-MS. Anal. Chem., 83 (2011) 5353–5360.
DOI: 10.1021/ac200780b

Maria Fernanda Giné, Ana Paula Packer,
On-Line Isotope Dilution and Inductively Coupled Plasma Mass Spectrometry: from Elemental to Species Quantification, J. Braz. Chem. Soc., 21/4 (2010) 575-589.
DOI: 10.1590/S0103-50532010000400002.

C.-K. Yang, P.-H. Chi, Y.-C. Lin, Y.-C. Sun, M.-H. Yang,
Development of an on-Line Isotope Dilution Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) Method for Determination of Boron in Silicon Wafers. Talanta, 80 (2010) 1222–1227.
DOI: 10.1016/j.talanta.2009.09.013

J. Heilmann,
S.F. Boulyga,
K.G. Heumann, Development of an Isotope
Dilution Laser Ablation ICP-MS Method for Multi-Element Determination in Crude and Fuel Oil Samples. J. Anal. At. Spectrom., 24 (2009) 385–390.
DOI: 10.1039/b819879a
 P. Rodríguez-González
P. Rodríguez-González,
J.M. Marchante-Gayón,
J.I. García Alonso,
A. Sanz-Medel,
Isotope dilution analysis for elemental speciation: a tutorial review, Spectrochim. Acta B, 60 (2005) 151–207.
doi: 10.1016/j.sab.2005.01.005
 J. Sabine Becker
J. Sabine Becker, Carola Pickhardt, W. Pompe,
Determination of Ag, Tl, and Pb in few milligrams of platinum nanoclusters by on-line isotope dilution in laser ablation inductively coupled plasma mass spectrometry, Int. J. Mass Spectrom., 237 (2004) 13–17.
DOI: 10.1016/j.ijms.2004.06.007

M. Tibi,
K.G. Heumann,
Isotope Dilution Mass Spectrometry as a Calibration Method for the Analysis of Trace Elements in Powder Samples by LA-ICP-MS. J. Anal. At. Spectrom., 18 (2003) 1076–1081.
DOI: 10.1039/b301835k

H. Scholze, E. Hoffmann, C. Lüdke, A. Platalla,
Analysis of Leaves by Using the Laser ICP-MS with Isotope Dissolution Method. Fresenius J. Anal. Chem., 355 (1996) 892–894. DOI:
10.1007/s0021663550892
 Related EVISA News :
Related EVISA News :