A group of Chinese researchers have developed a method for thallium speciation analysis based on separation by single-drop microextraction.
Background:
Thallium (Tl) is a rare and non-essential element which exhibits higher toxicity than Pb, Cd and Hg for humans. Exposure to Tl, even at low levels, can result in various health problems such as headache, paralysis, alopecia, vomiting, diarrhoea, cardiovascular diseases and kidney/liver failure. In nature, thallium usually exists as Tl(I) and Tl(III) ion forms that exhibit different bioavailability and toxicity properties. It was reported that the toxicity of Tl(III) is approximately several thousand times higher than that of Tl(I). Human exposure to Tl is mainly through water and food. Thus, it is of great importance to develop simple, sensitive and accurate methods for the determination of Tl species in food samples.
It is well known that sample preparation is the most critical step in speciation analysis of a given element. Sample preparation techniques for elemental forms include chromatographic and non-chromatographic separation methods. Modern techniques providing automated sample processing consists of the on-line coupling of a chromatographic separation system with a highly sensitive detection system, such as inductively coupled plasma mass spectrometry (ICP-MS).
However, sophisticated hyphenated systems such as HPLC-ICP-MS are expensive, call for skilled staffs and therefore are not available in many routine laboratories. For the speciation analysis of cases where the focus is on two simple species only and therefore the high separation power of chromatography is not required, non-chromatographic methods have attracted considerable attention.
Various non-chromatographic methods have been proposed for Tl species in real samples, including ion exchange, flow injection, solid phase extraction, dispersive solid phase extraction, dispersive liquid-liquid microextraction, dispersive micro-solid phase extraction and mixed-micelle cloud point extraction. Unfortunately, most of these methods are based on the selective detection of one of the two Tl species and the total thallium concentration. The total Tl concentration is obtained after converting all Tl to the determined species by redox reaction. Unfortunately, it is not that easy to convert species quantitatively at trace levels, and it calls for time-consuming operation.
The proposed solution: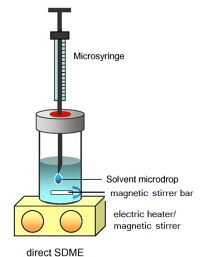
Figure: Schematic diagram of the
single-drop microextraction (SDME)
technique. |
A group of Chinese researchers is now proposing a method for the direct and sequential determination of both Tl(I) and Tl(III) species. The method is based on direct immersion single-drop microextraction (DI-SDME) where a solvent drop is formed on the tip of a syringe needle immersed into a stirred sample solution for the extraction of the target species from the sample matrix.
Two different extraction systems are used for the sequential extraction of the two species. 1-(2-pyridylazo)- 2-naphthol (PAN) is used for the complexation of Tl(III) and the Tl(III)-PAN complex is extracted into one 1-dodecanol drop. After the extraction of the Tl(III)-PAN complex, another nitrobenzene drop containing dicyclohexano-18-crown-6 (DCH-18-C-6) was immersed in the sample solution for the extraction of Tl(I). Finally, the extracts from TS-DI-SDME were analysed for their thallium concentration by graphite furnace atomic absorption spectrometry (GFAAS).
The method was optimized with respect to sample volume, pH of sample solution, drop volume, chelating reagent concentration, extraction solvent, sample temperature and reaction time.
Quantitative extraction was obtained for 20 min. extraction time and a solution temperature of 35°C for a sample volume of 20 mL. Under these optimized conditions, alkali metals, alkaline earth metals, trace transition elements and common anions had no impact on the determination. The optimized method was then used for the determination of Tl species in beverage samples after five-fold dilution with water.
The detection by GFAAS was also optimized with respect to the furnace temperature programming and the use of Pd(NO3)2 as a chemical modifier to prevent Tl losses during pyrolysis.
The final method had a linear working range of 0.04-40 ng/mL Tl and limits of detection of 1.9 ng/L and 2.5 ng/L for Tl(III) and Tl(I), respectively.
The authors concluded that their method has some merits such as low cost, simple operations and high extraction efficiency. They also believe that the proposed method may have good application potential for elemental species in environmental, food and biological samples.
Comment:The selection of non-chromatographic separation methods
for speciation analysis is often favoured with the argument of costs for
chromatographic equipment. The method described here calls for manual
operation of sample preparation consisting of sample aliquoting, sample
dilution, sample heating and stirring, drop immersion (2x), drop
collection (2x), drop dilution (2x), GFAAS sample cup loading (2x),
altogether more than one hour per sample. Mentioning the low cost of
such methods is totally ignoring the costs for manpower for such sample
processing.
Also, a processing time of 30 min under enhanced temperature for extracting a single species means to disturb the equilibrium between species in the sample in the direction of the extracted species. Non-chromatographic separation methods have a very limited separation power, especially prone to selectivity problems when one species is in great prevalence.
Both environmental and food analysis are highly regulated areas, where laboratories have to use validated methods ,
if not even restricted to standard methods. Methods calling for the
optimization of about ten parameters, such as the proposed method here,
call for careful method development, whenever the sample type is
changed. It is therefore not obvious to predict which type of
laboratories can make use of such method for which application area.
Please comment whether you can imagine to make use of such type of method or not!
Michael Sperling
 The original publication
The original publication

Shi-zhong Chen, Jun-tao Yan, Chun-lei Wang, Cheng-hao Zhang, Deng-bo Lu,
Determination of Tl(III) and Tl(I) in food samples with two-step direct immersion single-drop microextraction followed by graphite furnace atomic absorption spectrometry, J. Food Compos. Anal., 117 (2023) 104967.
DOI: 10.1016/j.jfca.2022.104967
 Related studies
Related studies

Juntao Yan, Chenghao Zhang, Chunlei Wang, Dengbo Lu, Shizhong Chen,
Solidified floating organic drop microextraction in tandem with syringe membrane miro-solid phase extraction for sequential detection of thallium (III) and thallium (I) by graphite furnace atomic absorption spectrometry, Arab. J. Chem., 15 (2022) 104335.
DOI: 10.1016/j.arabjc.2022.104335

I. Lopez-Garcia, M.J. Munoz-Sandoval, M. Hernandez-Cordoba,
Dispersive micro-solid phase extraction with a magnetic nanocomposite followed by electrothermal atomic absorption measurement for the speciation of thallium. Talanta 228 (2021) 122206.
DOI: 10.1016/j.talanta.2021.122206.

D.S. Krishna, N.N. Meeravali, S.J. Kumar,
A new sequential and simultaneous speciation analysis of thallium in coal effluents by graphite furnace atomic absorption spectrometry after a novel ligandless mixed micelle cloud point extraction. Int. J. Environ. Anal. Chem. 100/10 (2020) 1079–1093.
DOI: 10.1080/03067319.2019.1648643

Qingxiang Xiao, Atta Rasool, Tangfu Xiao, Philippe C. Baveye,
A modified method of separating Tl(I) and Tl(III) in aqueous samples using solid phase extraction, Chem. Cent. J., 12 (2018) 132.
DOI: 10.1186/s13065-018-0502-6

S. Rezabeyk, M. Manoochehri,
Speciation analysis of Tl(I) and Tl(III) after magnetic solid phase extraction using a magnetite nanoparticle composite modified with aminodibenzo-18-crown-6 functionalized MIL-101(Cr). Microchim. Acta 185/8 (2018) 365.
DOI: 10.1007/s00604-018-2881-8.

Z.D. Firouzabadi, A.M.H. Shabani, S. Dadfarnia, M.H. Ehrampoush,
Preconcentration and speciation of thallium by ferrofluid based dispersive solid phase extraction and flame atomic absorption spectrometry. Microchem. J. 130 (2017) 428–435.
DOI: 10.1016/j.microc.2016.10.025

E. Biadun, M. Sadowska, N. Ospina-Alvarez, B. Krasnodebska-Ostrega,
Direct speciation analysis of thallium based on solid phase extraction and specific retention of a Tl(III) complex on alumina coated with sodium dodecyl sulfate. Microchim. Acta., 183/1 (2016) 177–183.
DOI: 10.1007/s00604-015-1624-3

L.B. Escudero, C.B. Garcia, S.M. da Silva, J.H. Baron,
An eco-friendly cellular phase microextraction technique based on the use of green microalgal cells for trace thallium species determination in natural water samples. Anal. Methods, 7/18 (2015) 7480–7487.
DOI: 10.1039/c5ay01667c

B. Krasnodebska-Ostrega, M. Sadowska, K. Piotrowska, M. Wojda,
Thallium (III) determination in the Baltic seawater samples by ICP MS after preconcentration on SGX C18 modified with DDTC. Talanta 112 (2013) 73–79.
DOI: 10.1016/j.talanta.2013.03.059

Leticia B. Escudero, Rodolfo G. Wuilloud, Roberto A. Olsina,
Sensitive determination of thallium species in drinking and natural water by ionic liquid-assisted ion-pairing liquid–liquid microextraction and inductively coupled plasma mass spectrometry, J. Hazard. Mater., 244–245 (2013) 380– 386.
DOI: 10.1016/j.jhazmat.2012.11.057

Leticia B. Escudero, Paula Berton, Estefanía M. Martinis, Roberto A. Olsina, Rodolfo G. Wuilloud,
Dispersive liquid–liquid microextraction and preconcentration of thallium species in water samples by two ionic liquids applied as ion-pairing reagent and extractant phase, Talanta 88 (2012) 277– 283.
DOI: 10.1016/j.talanta.2011.09.068

Sonja Arpadjan, Pavleta Petrova, Jesper Knutsson,
Speciation analysis of thallium in water samples after separation/preconcentration with the Empore™ chelating disk, Int. J. Environ. Anal. Chem., 91/11 (2011) 1088-1099.
DOI: 10.1080/03067310903359476

M. Chamsaz, M.H. Arbab-Zavar, A. Darroudi, T. Salehi,
Preconcentration of thallium (I) by single drop microextraction with electrothermal atomic absorption spectroscopy detection using dicyclohexano-18-crown-6 as extractant system. J. Hazard. Mater. 167/1–3 (2009) 597–601.
DOI: 10.1016/j.jhazmat.2009.01.019

P.H. Pacheco, R.A. Gil, P. Smichowski, G. Polla, L.D. Martinez,
L-Tyrosine immobilized on multiwalled carbon nanotubes: a new substrate for thallium separation and speciation using stabilized temperature platform furnaceelectrothermal atomic absorption spectrometry. Anal. Chim. Acta 656/1–2 (2009) 36–41.
DOI: 10.1016/j.aca.2009.10.010

R.A. Gil, P.H. Pacheco, P. Smichowski, R.A. Olsina, L.D. Martinez,
Speciation analysis of thallium using electrothermal AAS following on-line pre-concentration in a microcolumn filled with multiwalled carbon nanotubes. Microchim. Acta 167/3–4 (2009) 187–193.
DOI: 10.1007/s00604-009-0241-4

N.N. Meeravali, S.J. Jiang,
Ultra-trace speciation analysis of thallium in environmental water samples by inductively coupled plasma mass spectrometry after a novel sequential mixed-micelle cloud point extraction. J. Anal. Spectrom. 23/4 (2008) 555–560.
DOI: 10.1039/b718149c

S. Dadfarnia, T. Assadollahi, A.M.H. Shabani,
Speciation and determination of thallium by on-line microcolumn separation/preconcentration by flow injection flame atomic absorption spectrometry using immobilized oxine as sorbent. J. Hazard. Mater. 148/1–2 (2007) 446–452.
DOI: 10.1016/j.jhazmat.2007.02.059
 Related EVISA Resources
Related EVISA Resources
 Brief summary: Non-chromatographic separation techniques for speciation analysis
Brief summary: Non-chromatographic separation techniques for speciation analysis
 Related EVISA News (Newest first)
Related EVISA News (Newest first)
 November 17, 2019: Determination of Thallium Speciation in Water Samples by HPLC-ICP-MS
November 17, 2019: Determination of Thallium Speciation in Water Samples by HPLC-ICP-MS
last time modified: September 18, 2024