|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
|
Often called Student’s t-test. A statistical
test of significance in which the difference between two mean values is
tested. The null hypothesis is that there is no difference between the
two means. The test is carried out by calculating a t-value, then
comparing the calculated t-value with a critical t-value which is
obtained from a statistics table. If the calculated t-value is greater
than the critical t-value, the null hypothesis is rejected; this means
that a statistically significant or real difference exists between the
mean values being compared. If the calculated t-value is less than the
critical t-value, the null hypothesis stands, therefore no difference
has been observed between the two mean values.
|
| |
|
An arrangement in which ions are subjected to two or more sequential
stages of analysis (which may be separated spatially or temporally)
according to the quotient mass- charge. Inbetween analysers, gas phase
reactions may be initiated, aiding in the interpretation of
fragmentation patterns.
|
| |
|
Tandem Mass Spectrometry, usually referred to as MS/MS, involves the use of 2 or more mass analyzers. It is often used to analyze individual components in a mixture. This technique adds specificity to a given analysis.
The basic idea of MS/MS is a selection of a m/z of a given ion formed in the ion source, and subject this ion to fragmentation, usually by collision with inert gas (eg. Argon). The product ions are then detected. This is a powerful way of confirming the identity of certain compounds and determining the structure of unknown species. So MS/MS is a process that involves 3 steps: ionization-mass selection-mass analysis.
MS/MS could be performed on instruments such as triple quadrupole (QQQ), ion trap, time of flight, fourier transform, etc... The triple quadrupole is the most frequently used mass spectrometer for MS/MS, perhaps because of the cost and ease of use among other factors.
|
| |
|
|
The tandem MS is an instrumental arrangement in which ions
are subjected to two or more sequential stages (which may be separated
spatially or temporally) of analysis according to the mass-to-charge
ratio. The study of ions by means of two stages of mass analysis is
termed mass spectrometry/mass spectrometry (MS/MS)
|
| |
|
A separation technique that transfers components of one system (stream) into another. The stream thge product is being extracted from acrosses the stream that the product is being transferred to multiple times.
|
| |
|
|
Used in proficiency testing to
designate the correct value, usually estimated by the mean of all
participant responses, after removal of outliers, or by the mean
established by definitive or reference methods.
|
| |
|
|
A Taylor cone refers to the conical shape of the liquid emanating from a
capillary under high voltage such as in electrospray or other
electrohydrodynamic spray proces.
|
| |
|
Tetraethyllead (common name tetraethyl lead), abbreviated TEL, is an organolead compound with the formula (CH3CH2)4Pb. Once a common antiknock additive/octane booster in gasoline (petrol), TEL usage was largely discontinued because of the toxicity of lead and its deleterious effect on catalytic converters. It is still used as an additive in aviation fuel for piston engine-powered aircraft.
  
|
| |
|
A procedure in which the temperature of the column is changed systematically during a part or the whole of the separation.
Source: IUPAC
|
| |
|
|
an agent capable of causing abnormalities in a developing foetus; that is, causing birth defects
|
| |
|
|
Potential to cause structural malformations or defects in the offspring
|
| |
|
The three-dimensional folding (its normal state) of a polypeptide chain in a protein molecule.
|
| |
|
|
Tetramethylammonium hydroxide (TMH) is an effective etchant and chelating agent ideal for removing residue and contaminants from semiconductor and electronic components. It also is often used to solubilze biological materials by alkaline hydrolysis.
Molecular formula: C4H13NO.5H2O
MW: 181.22972
|
| |
|
Ionization of particles brought about by a high temperature—for example, emission of ions from an adsorbed layer on an incandescent metal surface.
Source: IUPAC
|
| |
|
An uncatalysed bond cleavage resulting from exposure of a compound to a raised temperature.
|
| |
|
|
Thermospray ionization is a method of ion formation in which a liquid is
flowed through a heated capillary to produce a spray of droplets and
solvent vapor. Ions are formed due to the statistical imbalance of
charges in the droplets or by a heated filamen.
|
| |
|
|
Thermospray nebulization is a method of aerosol formation in which a liquid is
flowed through a heated capillary to produce a spray of droplets and
solvent vapor.
|
| |
|
Thimerfonat (Timerfonat) is an organic mercury compound used since the 1930's as a preservative for some vaccines.
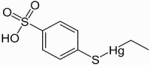 Other names for Timerfonat: Other names for Timerfonat:
- Ethyl-(4-sulfophenyl) sulfanylquecksilber
- Natriumtimerfonat (Na-Salz)
- Natriumthimerfonat (Na-Salz)
Formula: C8H10HgO3S2
CAS Number: 33305-56-5 (5964-24-9 Na salt)
|
| |
|
|
Thimerosal is an organometallic compound containing mercury, commonly used as a preservative. It is found in cosmetics, including makeup removers, eye moisturizers, and mascaras. Some other places in which Thimerosal may be found include: soap-free cleansers; nose, eye, and ear drops; eye ointments; topical medications; and antiseptic sprays. It is also found in cleaning fluids that are used for contact lenses. Thimerosal is also used widely as a preservative in antitoxins, tuberculin tests, desensitization solutions and vaccines, most controversially including those widely administered to children. Its chemical formula is C9H9HgNaO2S. It has been in use since the 1930s.
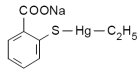 Other names for thimerosal: Other names for thimerosal:
Thimerosol, Merthiolate, Sodium ethylmercurithiosalicylate, Mercurothiolate, Thiomersal, Thiomersalate, Thiomersalan, Ethyl (2-mercaptobenzoato-S) mercury sodium salt, [(o-carboxyphenyl)thio] Ethylmercury sodium salt
|
| |
|
|
Liquid chromatography carried out in a layer of adsorbent spread on a support, e.g. a glass plate.
|
| |
|
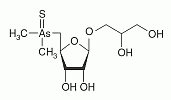 Thio-arsenosugar-glycerol has been found in mussel samples. Thio-arsenosugar-glycerol has been found in mussel samples.
|
| |
|
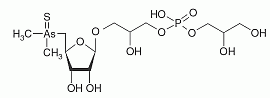 Thio-arsenosugar-phosphate has been found in mussel samples. Thio-arsenosugar-phosphate has been found in mussel samples.
|
| |
|
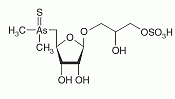 Thio-arsenosugar-sulfate has been identified as a constituent of aqueous and methanol extracts from the gonad and the muscle of the great scallop. Thio-arsenosugar-sulfate has been identified as a constituent of aqueous and methanol extracts from the gonad and the muscle of the great scallop.
|
| |
|
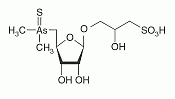 Thio-arsenosugar-sulfonate has been identified as a constituent in aqueous and methanol extracts from the gonad and the muscle of the great scallop. Thio-arsenosugar-sulfonate has been identified as a constituent in aqueous and methanol extracts from the gonad and the muscle of the great scallop.
|
| |
|
|
An aqueous species, such as H2AsO3S− and H2AsS2O2−, that contains pentavalent arsenic and sulfide (compare with arsenate, thioarsenic, thioarsenite, and methylthioarsenate).
|
| |
|
|
An aqueous species, such as HAs3S62− and H2AsO3S−, that contains sulfide and arsenic, either as arsenide (As3−), As3+, or As5+ (compare with arsenosulfide, methylthioarsenate, thioarsenate, and thioarsenite).
|
| |
|
|
An aqueous species, such as H2As3S6−, that contains trivalent arsenic and sulfide (compare with arsenite, thioarsenic, thioarsenate, and methylthioarsenate).
|
| |
|
|
An organic compound or chemical species containing a sulfhydryl (–SH) group. Methanethiol (CH3SH) is an example of a thiol.
|
| |
|
|
A protein containing a Se-Cysteine residue that regulates intracellular redox reactions (including reduction of thioredoxin) and factors into repair mechanisms in DNA synthesis.
|
| |
|
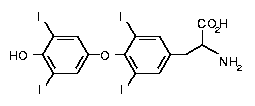 The
major form of thyroid hormone in the blood is thyroxine (T4, see left).
This prohormone is converted to the active T3 within cells by deiodinases.
Thyroxine (3,5,3',5'-tetraiodothyronine) is produced by follicular
cells of the thyroid gland by attaching four iodine atoms to the ring
structures of tyrosine molecules The
major form of thyroid hormone in the blood is thyroxine (T4, see left).
This prohormone is converted to the active T3 within cells by deiodinases.
Thyroxine (3,5,3',5'-tetraiodothyronine) is produced by follicular
cells of the thyroid gland by attaching four iodine atoms to the ring
structures of tyrosine molecules
|
| |
|
Observing only a portion of a datastream by selecting time intervals, subsets of the overall datastream, for recording.
|
| |
|
The TOF MS exploits the fact that ions of different mass-charge need
different times to travel through a certain distance in a field- free
region after they have all been initially given the same kinetic energy or same impulse.
|
| |
|
Tin is a chemical element in the periodic table that has the symbol Sn (Latin: stannum) and atomic number 50. This silvery, malleable poor metal that is not easily oxidized in air and resists corrosion is found in many alloys and is used to coat other metals to prevent corrosion. Tin is obtained chiefly from the mineral cassiterite, where it occurs as an oxide. It is the classic alloying metal to make bronze.
Elemental tin is an essential nutrient, needed in very small amounts. However, certain organic tin compounds, organotin, such as triorganotins (see tributyltin oxide) are toxic and are used as industrial fungicides and bactericides.
|
| |
|
A measured sample. (To draw a measured, representative sample from a larger amount is to titrate)
|
| |
|
|
A TDI is an estimate of the amount of a substance in air, food or drinking
water that can be taken in daily over a lifetime without appreciable health
risk. TDIs are calculated on the basis of laboratory
toxicity data to which
uncertainty
factors are applied.
TDIs are used for substances that do not have a reason to be found in food (as
opposed to substances that do, such as additives, pesticide residues or
veterinary drugs in foods- see also Acceptable Daily Intake : ADI).
|
| |
|
|
Top-down proteomics is a method of protein identification that uses the m/z selection of intact proteins followed by fragmentation and m/z separation in a second stage of mass spectrometry. Mixtures of proteins must first be separated by liquid chromatography or other separation method prior to mass spectrometry.
|
| |
|
|
"Tortured phrases" is a term coined by researchers to describe strange, incorrect, or unnatural substitutions of technical terms that often appear in low-quality, AI-generated or paper-mill–generated scientific texts.
Here are some examples:
Artificial Intelligence & Computing- Artificial Intelligence (AI) → counterfeit consciousness, fake intelligence
- Machine Learning → apparatus learning, mechanical acquiring
- Deep Learning → profound learning, deep tutoring
- Neural Network → neuron net, brain organization
- Convolutional Neural Network (CNN) → folded brain arrangement, convoluted neuron grid
- Random Forest → arbitrary woodland, stochastic trees
- Support Vector Machine (SVM) → prop-up vector contraption, help outline motor
- Gradient Descent → incline reduction, slope fall
Mathematics & Statistics- Mean Square Error (MSE) → average squared fault, mean square slip-up
- Principal Component Analysis (PCA) → main constituent examination, fundamental section review
- Logarithm → log book, proportionate mathematics
- Eigenvalue/Eigenvector → characteristic worth, own vector
- Regression Analysis → backslide examination
Engineering & Physics- Signal-to-noise ratio (SNR) → flag-to-commotion proportion, signal vs. turbulence
- Finite Element Method (FEM) → restricted component strategy
- Quantum Entanglement → quantum perplexity, quantum complexity
- Thermodynamics → warmth elements, heat force study
Biology & Medicine- Gene Expression → quality articulation
- Polymerase Chain Reaction (PCR) → polymerase loop response
- Molecular Docking → atomic harborage, molecule mooring
- Placebo Effect → fake treatment impact
- Antimicrobial Resistance (AMR) → microbe opposition
Why These Are Red Flags- They signal paper mills or paraphrased plagiarism.
- They reduce scientific clarity and searchability.
- No genuine researcher would use these phrasings in proper context.
|
| |
|
|
Total atmospheric mercury (TAM) is operationally defined as the sum of gaseous elemental mercury (GEM), gaseous oxidized inorganic mercury (GOIM), which is also called reactive gaseous mercury (RGM), and total filterable mercury (TFM).
|
| |
|
|
Phosphorus form present in water that had been filtered through 0.45 µm membrane* then subjected to oxidative digestion process (APHA, 1989). Includes both dissolved organic and inorganic P.
|
| |
|
TGM is a mercury fraction operationally defined as species passing
through a 0.45 µm filter or some other simple filtration device such as
quartz wool plugs, and which are collected on gold or other collection
material. TGM is mainly composed of elemental mercury vapor with minor
fractions of other volatile species such as HgCl2, CH3HgCl or (CH3)2Hg.
|
| |
|
|
A total ion chromatogram is a plot of the total ion signal in each of a
series of mass spectra that are recorded as a function of
chromatographic retention time. Care should be taken to avoid confusion between the abbreviations for total ion current and total ion chromatogram.
|
| |
|
|
The total ion profile is a plot of the total ion signal from a series of
mass spectra that are recorded as a function of time, for example
chromatogram, electropherogram, flow injection analysis, or other
time-dependent samplin.
|
| |
|
|
Includes all forms of mercury
|
| |
|
TPM consiusts of mercury bound or adsorbed to atmospheric particulate
matter. Several different components are possible: Hg° or RGM adsorbed
to the particle surface, or divalent mercury species chemically bound
to the particle or integrated into the particle itself.
|
| |
|
|
Sum of organic and inorganic forms of phosphorus. TP is measured on unfiltered water samples that has been subjected to oxidative destruction of organic matter (APHA, 1989)..
|
| |
|
The total-ion current (TIC) trace is the sum of the relative abundances of all the ions in each mass spectrum plotted against the time (or number of scans) in a data collection sequence. For example, the total-ion current trace in a GC/MS run is analogous to the output of a single-channel gas chromatography detector. The trace allows eluted peaks to be identified by an increase in the total-ion current over background. The scans corresponding to the eluted peak are averaged together to create the mass spectrum of the sample corresponding to that peak.
  
|
| |
|
|
Substance causing injury to living organisms as a result of physicochemical interactions.
|
| |
|
|
This is the preferred term for a substance that is considered to be toxic under circumstances which are thought likely to happen.
|
| |
|
|
The process of interaction of chemical substances with target sites and the subsequent reactions leading to adverse effects
|
| |
|
|
Toxicogenomics combines toxicology, genetics, molecular biology, and environmental health to elucidate the response of living organisms to stressful environments or toxic agents. Likewise, new drug candidates can be screened through a combination of gene expression profiling and toxicology to understand gene response and possibly predict safety.
|
| |
|
|
Toxicokinetics is the process of the uptake of potentially toxic substances by the body, the biotransformation they undergo, the distribution of the substances and their metabolites in the tissues, and the elimination of the substances and their metabolites from the body (Nordberg et al., 2004).
|
| |
|
|
Poisonous substance produced by a biological organism such as a microbe, animal or plant.
|
| |
|
|
Traceability is a term from metrology and is defined as the property of the result of a measurement or the value of a standard whereby it can be related to stated references, usually national or international standards, through an unbroken chain of comparisons all having stated uncertainties.
Source: International Vocabulary of Basic and General Terms in Metrology, International Standardization Organisation, Geneva, Switzerland, 2nd edition, 1993
|
| |
|
|
Substance which can be tracked through one or more reactions or systems, often by detecting an incorporated isotope (isotope labeling).
|
| |
|
|
The interface of a hyphenated technique connecting the effluent from a gas-chromatograph with the inlet of an element detection system. In order to avoid losses by condensation etc. external heating is applied to the transfer line.
|
| |
|
|
Transferrin is a glycoprotein with a relative mass of about 80,000 Da
containing two high affinity binding sites. Plasma transferrin is
involved in transporting iron from the alimentary tract to the tissues
of the body. Its extremely high binding affinity (1022 dm3mol-1) means
that it can also outcompete many microorganisms for iron, acting as a
bacteriostat. The two metal-binding sites on transferrin are similar but not
identical and function independently. In the absence of iron
transferrin is capable of binding other metal ions such as aluminium,
chromium, cobalt, copper, manganese, vanadium as well as plutonium and
europium. Similar proteins are found in milk (lactoferrin) and eggs
(ovotransferrin).
|
| |
|
|
Signals that are short in duration and changing with the measurement time, such as created by sample introduction systems like flow injection, electrothermal vaporization or laser ablation are called transient signals. In order to evaluate such transient signals, the signal evaluation routine should be able to precisely follow and record the time-dependent signal level, rather than to integrate the signal.
|
| |
|
|
A transition element is an element whose atom has an incomplete d-sub-shell, or which gives rise to a cation or cations with an incomplete d-sub-shell.
The First Transition Series of elements is Sc, Ti, V, Cr, Mn, Fe, Co,
Ni, Cu. The Second and Third Transition Series are similarly derived:
these include the lanthanoids (lanthanides) and actinoids (actinides)
respectively which are designated inner (or f) transition elements of their respective Periods in the Periodic Table.
|
| |
|
Transfer of a methyl group from one compound to another. If the process is biologically mediated, it is also called biomethylation.
|
| |
|
|
Tributyltin compounds are a group of organotin compounds containing the (C4H9)3Sn (TBT) moiety, such as tributyltin hydride or tributyltin oxide. They are the main active ingredients in certain biocides used to control a broad spectrum of organisms, and these compounds are being considered for inclusion in the Rotterdam Convention.
|
| |
|
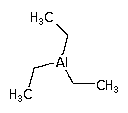 Triethylaluminium (TEAl) can be used as a precursor for aluminum oxide deposition using the MOCVD process ( Metal Organic Chemical Vapor Deposition ) and in support of semi-conductor manufacturing. Formula: (C2H5)3Al
CAS Number : 97-93-8
Other names: Aluminium triethyl; TEA
|
| |
|
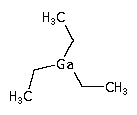 In semiconductor manufacturing, triethyl gallium (TEGa) is used in MOCVD to deposit GaAs or GaN layers.
(C2H5)3Ga ; Gallium triethyl
CAS Number : 1115-99-7
|
| |
|
|
(CH3)3Bi - trimethylbismuthine, a volatile metalalkyl compound, has been identified in a variety of anthropogenic gases, e.g. landfill gas and sewage sludge digester gas. Biomethylation of inorganic bismuth compounds is performed by different microorganisms, such as bacteria.
|
| |
|
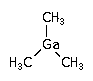 In semiconductor manufacturing, trimethyl gallium (TMGa) is used in OrganoMetallic Vapor Phase Epitaxy (MOCVD) for the deposition of epitaxial GaAs or GaN layers.
(CH3)3Ga ; Gallium trimethyl
CAS Number : 1445-79-0
|
| |
|
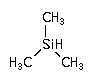 (CH3)3-SiH ; Trimethylsilane (CH3)3-SiH ; Trimethylsilane
CAS Number : 993-07-7
|
| |
|
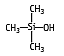 Trimethylsilanol has been the most abundant compound determined in waste composting gas samples, accompanied by several siloxanes. IUPAC name: Trimethylhydroxysilane
Molecular formula: C3H10OSi
Molecular weight: 90.20
|
| |
|
|
(CH3)3Sb - Trimethylstibine, also called trimethyl antimony, is generated through methylation from organic and an inorganic salt of antimony by microorganisms. The volatile metalloid compound has been identified in a variety of anthropogenic gases, e.g. landfill gas and sewage sludge digester gas.
|
| |
|
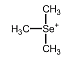 The organoselenium species trimethylselenonium has been reported to be a Se-metabolite in rat urine (especially at enhanced exposure). In urine of healthy humans this species has not been unambigously confirmed. The organoselenium species trimethylselenonium has been reported to be a Se-metabolite in rat urine (especially at enhanced exposure). In urine of healthy humans this species has not been unambigously confirmed.
|
| |
|
|
The triple filament method
is a surface ionization method used for TIMS wherein 3
filaments are used. Two filaments are used to heat and vaporize the
sample and 1 filament, at a very high temperature, is used to ionize the
sample.
|
| |
|
One of the most popular types of tandem MS instrument is the triple quadrupole mass spectrometer, invented at Michigan State University by Richard A. Yost (now a chemistry professor at the University of Florida, Gainesville) and chemistry professor Christie G. Enke (now at the University of New Mexico, Albuquerque). James D. Morrison of Latrobe University, Melbourne, Australia, helped Yost and Enke reduce the technique to practice.
|
| |
|
|
An MS/MS device created by connecting 3 quadrupole mass spectrometers in
sequence. The first instrument is used for precursor ion selection, the
second as a collision tube for CID or SID, and the third for detection
of fragment ions created by dissociation of precursor ions. CID spectra
of low-energy collisions can be obtained with this instrument.
|
| |
|
A group of organisms that occupy the same position in a food chain-
  
|
| |
|
|
A mixture formed by the hydrolysis of a peptide or protein, or a mixture of these, using the enzyme trypsin. Cleavage of the peptide chain occurs on the C-terminal side of arginine and lysine residues to give a series of peptides which can be identified by sequencing or mass mapping to lead to identification of the original protein. The technique is used extensively in proteomics.
|
| |
|
|
Thermospray flame furnace atomic absorption spectrometry: A type of atomic absorption spectrometry where the liquid sample is forced through a heated metal capillary tip before atomisation in a flame.
|
| |
|
An ion source for mass spectrometry capable of producing either
elemental or molecular information depending on the plasma power
selected.
|
| |
|