Researchers from the University of Münster have developed analytical methods for the simultaneous determination of gadolinium-based contrast agent Magnevist (Gd-DTPA) and its transmetalation products in complex clinical samples.
Background:Gadolinium chelates are used as contrast agents for medical magnetic resonance imaging (MRI) since the 80's because of the excellent magnetic properties of gadolinium. About 20,000,000 patients per year are investigated by this diagnostic tool, each one receiving typically around 1.2 g of gadolinium. Since free gadolinium is highly toxic, gadolinium-based contrast agents (GdCA) contain the element in highly stable complexes with linear or macrocyclic polyaminocarboxylic acid ligands. In general, these Gd-complexes show good tolerance in humans and fast clearance time so that adverse reactions are rarely observered.
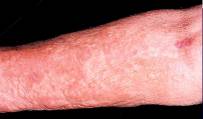
However, clearance from the patient's body is hampered in renal impaired patients and in some few cases a newly observed disease called nephrogenic systemic fibrosis (NSF) has been diagnosed for such patients after the use of Gd-enhanced MRI. Until now, the patho-mechanism through which the application of GdCAs results in NSF are not fully understood.
Photo: NSF is a life threatening and life changing disease. It causes thickening and fibrosis of the skin, organs and other tissues.The new study:The
researchers from the University of Münster based their work on the following hypotheses:
- Clearance of GdCAs in renal-impaired patients is reduced or even abolished leading to prolonged retention of the GdCA in the body.
- GdCA decomposition in the body is enhanced because of the longer clearance time.
- Gd is set free from the GdCA by exchange with another metal such as iron, copper or zinc.
In order to study such transmetalation reaction, the research group developed analytical methods based on hyphenated techniques providing the analytical detection power to investigate the real situation in the complex matrix of blood plasma. Capillary electrophoresis was used for the separation of the GdCA and its potential transmetalation products. Electrospray ionization time-of-flight mass spectrometry (ESI-tof-MS) was used for detection and identification.
Blood plasma samples from ten MRI patients were analysed and incubation experiments mimicking the situation in renal-impaired patients were caried out.
No transmetalation compounds for Gd-DTPA were observed in blood plasma samples from patients with normal renal function. Also, transmetalation compounds were not observed from incubated blood samples, when only the Gd-CA was added. However, remarkable amounts of Fe-DTPA were found in samples incubated with supplementary iron. Whether parenteral iron supplements usually applied for renal-impaired patients will also show such transmetalation in-vivo remains to be studied.
 The original study
The original study
Jens Künnemeyer, Lydia Terborg, Sascha Nowak,
Lena Telgmann, Faruk Tokmak, B.K. Krämer, Aandreas Günsel, Gerhard A. Wiesmüller, J. Waldeck, C. Bremer,
Uwe Karst,
Analysis of the Contrast Agent Magnevist and Its Transmetalation Products in Blood Plasma by Capillary Electrophoresis/Electrospray Ionization Time-of-Flight Mass Spectrometry, Anal. Chem., 81/9 (2009) 3600-3607.
DOI: 10.1021/ac8027118 Related studies:
Related studies:
Jens Künnemeyer, Lydia Terborg, Sascha Nowak, Andy Scheffer,
Lena Telgmann, Faruk Tokmak, Andreas Günsel, Gerhard Wiesmüller, Stephan Reichelt,
Uwe Karst,
Speciation Analysis of Gadolinium-Based MRI Contrast Agents in Blood Plasma by Hydrophilic Interaction Chromatography/Electrospray Mass Spectrometry, Anal. Chem. , 80/21 (2008) 8163-8170.
DOI: 10.1021/ac801264j
Clark D. Wiginton, Brent Kelly, Aytekin Oto, Mary Jesse, Patricia Aristimuno, Randy Ernst, Gregory Chaljub,
Gadolinium-Based Contrast Exposure, Nephrogenic Systemic Fibrosis, and Gadolinium Detection in Tissue, Am. J. Roentgenol., 190 (2008) 1060-1068.
DOI: 10.2214/AJR.07.2822
Marc Port, Jean-Marc Idée, Christelle Medina, Caroline Robic, Monique Sabatou, Claire Corot,
Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review, BioMetals, 21 (2008) 469-490.
DOI: 10.1007/s10534-008-9135-x
Thomas Frenzel, Philipp Lengsfeld, Heiko Schirmer, Joachim Hütter, Hanns-Joachim Weinmann,
Stability of Gadolinium-Based Magnetic Resonance Imaging Contrast Agents in Human Serum at 37°C, Invest. Radiol., 43/12 (2008) 817.
DOI:
10.1097/RLI.0b013e3181852171
Petr Hermann, Jan Kotek, Vojtech Kubícek, Ivan Lukes,
Gadolinium(III) complexes as MRI contrast agents: ligand design and properties of the complexes, Dalton Trans., (23) (2008) 3027-3047.
DOI: 10.1039/b719704g
Valeria Loreti,
Jörg Bettmer,
Determination of the MRI contrast agent Gd-DTPA by SEC-ICP-MS, Anal. Bioanal. Chem., 379/7-8 (2004) 1050-1054.
DOI: 10.1007/s00216-004-2700-4

Ernö Brücher,
Kinetic Stabilities of Gadolinium(III) Chelates Used as MRI Contrast Agents, Top. Curr. Chem., 221 (2002) 103-122.
DOI: 10.1007/3-540-45733-X_4  Related Information
Related Information FDA: December 12, 2007: Important Drug Warning for Gadolinium-Based
FDA: December 12, 2007: Important Drug Warning for Gadolinium-Based
Contrast Agents FDA: Information on Gadolinium-Containing Contrast Agents
FDA: Information on Gadolinium-Containing Contrast Agents Related EVISA Resources
Related EVISA Resources  Brief Summary: ESI-MS: The tool for the identification of chemical species
Brief Summary: ESI-MS: The tool for the identification of chemical species Link Database: All about gadolinium
Link Database: All about gadolinium Brief summary: Speciation analysis for the study of metallodrugs and their biomolecular interactions
Brief summary: Speciation analysis for the study of metallodrugs and their biomolecular interactions Related News (newest first)
Related News (newest first)
 March 4, 2015: Detection of Gd-based contrast agent in the skin of a patient eight years after administration
March 4, 2015: Detection of Gd-based contrast agent in the skin of a patient eight years after administration  October 29, 2012: Identification and quantification of potential metabolites of Gd-based contrast agents
October 29, 2012: Identification and quantification of potential metabolites of Gd-based contrast agents  October 18, 2012: The behavior of Gd-based contrast agents during wastewater treatment
October 18, 2012: The behavior of Gd-based contrast agents during wastewater treatment September 15, 2010: US FDA Announces Gadolinium-Based MRI Contrast Agent Warning
September 15, 2010: US FDA Announces Gadolinium-Based MRI Contrast Agent Warning March 25, 2010: Publication on the separation of Gd-based contrast agents awarded
March 25, 2010: Publication on the separation of Gd-based contrast agents awarded May 4, 2009: Gadolinium speciation analysis in search for the cause of nephrogenic systemic fibrosis (NSF)
May 4, 2009: Gadolinium speciation analysis in search for the cause of nephrogenic systemic fibrosis (NSF)  April 14, 2009: Gadolinium-based MRI contrast agents found intact in the outlet of a waste water treatment plant
April 14, 2009: Gadolinium-based MRI contrast agents found intact in the outlet of a waste water treatment plant
last time modified: April 19, 2025