A group of Chinese researchers developed a mild extraction method that enabled them to catch the relatively unstable mercurous species from different environmental samples such as fly ash, soil, and plant material.
Background:Mercury (Hg) is a highly toxic metal, and its ubiquity in the environment and long-range transport make it a global pollutant. Elemental Mercury [Hg(0)], mercuric Hg [Hg(II)] and methylmercury [CH3Hg+] are three well-known Hg species in the environment with different toxicity and transformation pathways between them. The transformation between Hg(0) and Hg(II) therefore play an import role for the transport, bioavailability and toxicity of Hg.
Since most of the redox processes transforming Hg(0) to Hg(II) involve single-electron transfer, mercurous Hg [Hg(I)] should be an important intermediate. However, Hg(I) is unstable in water and will rapidly disproportionate into Hg(0) and Hg(II).
Unfortunately, available analytical methods for mercury speciation analysis cannot fully meet the requirements for the determination of the unstable Hg(I) species occurring at low concentrations. While direct speciation techniques such as extended X-ray absorption fine structure (EXAFS), X-ray photoelectron spectroscopy, X-ray diffraction, and Raman spectroscopy could be used for Hg(I) species identification, their poor sensitivity limits their application for environmental sample analysis. Speciation techniques based on volatilization, thermal desorption or gas chromatography are prone to significant oxidation during separation. Liquid-phase separation processes do not require a high temperature or derivatization, and thus could avoid such transformation. However, such techniques require a liquid sample and extraction procedures using heating, or assistance through ultrasound or microwave irradiation may change the speciation of Hg. Therefore, a mild extraction and analytical method for Hg(I) is needed to examine its presence in environmental solid materials.
The new study:A group of Chinese Researchers aimed at closing the gap by developing the speciation analysis of various solid matrices for the occurrence of Hg(I) based on the hyphenation of HPLC and ICP-MS. They first screened various sulphur-containing complexation agents and some extraction methods using different agitation modes for their extraction efficiency and effects on the Hg speciation. When using 0.2% mercaptoethanol as complexation agent for Hg, Hg(I) was not reduced in 8h. The comparison of different assisted energy input modes revealed that ultrasound converted 89% of Hg(I) to Hg(0) within 30 min. Similar losses of Hg(I) were observed by heating or microwave irradiation. To avoid such conversion, mechanical shaking was selected as agitation mode for extraction of Hg(I) from solid matrices.
Separation of Hg species (Hg(I), Hg(II) and CH3Hg(II)) was achieved by using C18 column-based reverse-phase chromatography with 0.5% mercaptoethanol (ME) as mobile phase. Using ME extraction and RP-HPLC-ICP-MS for Hg speciation analysis, various solid matrices, including phosphorus powder in used fluorescent lamps, desulphurised gypsum and fly ash from a coal-fired power plant, soil and plants from a Hg mining area, atmospheric PM2.5 from Beijing, and Hg(II)-exposed marine microalgae were tested for the occurrence of Hg(I). Hg(I) was found in all type of matrices tested.
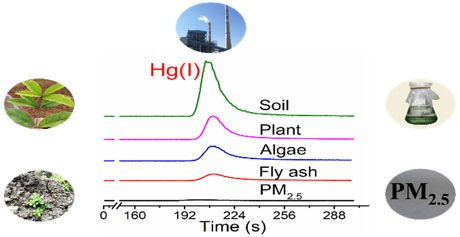
These results suggest that Hg(I) is an important intermediate in the reduction and oxidation of Hg(II) and Hg(0). The authors also speculate that the presence of Hg(I) may significantly influence the photoreduction kinetics and the reducible Hg pool, especially in chloride-containing environments. They conclude, that further studies are warranted to examine its transport (e.g., evasion) and transformation (e.g., methylation) in the environment, as well as its bioavailability and toxicity to living organisms.
 The Original study
The Original study
Ying Wang, Guangliang Liu, Yanbin Li, Yanwei Liu, Yingying Guo, Jianbo Shi, Ligang Hu, Yong Cai, Yongguang Yin, Guibin Jiang,
Occurrence of Mercurous [Hg(I)] Species in Environmental Solid Matrices as Probed by Mild 2‑Mercaptoethanol Extraction and HPLC-ICP-MS Analysis, Environ. Sci. Technol. Lett., 7 (2020) 482−488. DOI:
10.1021/acs.estlett.0c00329 Used techniques and instrumentation:
Used techniques and instrumentation:
 Agilent Technologies Inc. - 1100 HPLC
Agilent Technologies Inc. - 1100 HPLC
 Related studies (newest first)
Related studies (newest first)

C. Han, W. Wang, F. Xie, G. Wang, A. Volinsky, M. Gekhtman,
Study on pure mercurous chloride leaching with sodium thiosulfate. Russ. J. Non-Ferr. Met., 59/6 (2018) 589−595.
DOI: 10.3103/S1067821218060056

H. Cheng, C. Wu, L. Shen, J. Liu, Z. Xu,
Online anion exchange column preconcentration and high performance liquid chromatographic separation with inductively coupled plasma mass spectrometry detection for mercury speciation analysis. Anal. Chim. Acta, 828 (2014) 9−16.
DOI: 10.1016/j.aca.2014.04.042

I. Cattani, S. Spalla, G.M. Beone, A.A.M. Del Re, R. Boccelli, M. Trevisan,
Characterization of mercury species in soils by HPLC-ICP-MS and measurement of fraction removed by diffusive gradient in thin films. Talanta, 74/5 (2008) 1520−1526.
DOI: 10.1016/j.talanta.2007.09.029

C.M. do Valle, G.P. Santana, C.C. Windmöller,
Mercury conversion processes in Amazon soils evaluated by thermodesorption analysis. Chemosphere, 65/11 (2006) 1966−1975.
DOI: 10.1016/j.chemosphere.2006.07.001

F. Raofie, P.A. Ariya,
Product study of the gas-phase BrO-initiated oxidation of Hg0: evidence for stable Hg1+ compounds. Environ. Sci. Technol., 38/16 (2004) 4319−4326.
DOI: 10.1021/es035339a

Donald C. Wigfield, Sherry L. Perkins,
The Misbehaviour of Mercury(I) Ions in Cold-Vapour Atomic Absorption Spectrometry, Anal. Chim. Acta, 167 (1985) 419-424.
DOI: 10.1016/S0003-2670(00)84450-5
 Related EVISA Resources
Related EVISA Resources
last time modified: January 11, 2025