A team of German researchers has developed an innovative method utilizing IC-ICP-OES for the quantification of inositol phosphates. This method successfully separated 28 isomers within 33 minutes.
Background:Phytic acid, also known as inositol hexaphosphate, is the principal storage form of phosphorus in many plant tissues, particularly in bran, seeds, legumes, cereals, and grains. Structurally, phytic acid consists of an inositol ring esterified with six phosphate groups, making the phosphate unavailable for absorption by non-ruminant animals. In contrast, ruminants can digest phytate due to the phytase enzyme produced by rumen microorganisms, which hydrolyses the inositol-phosphate bond. Over a wide pH range, phytic acid exists as a negatively charged molecule, often forming insoluble complexes with various minerals such as iron, zinc, calcium, and proteins. This significant chelation potential raises concerns about its impact on the bioavailability of essential minerals, categorizing it as an antinutritional factor.
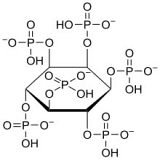
The hexavalent phytate anion
|
Lower inositol polyphosphates, which are inositol esters with fewer than six phosphates, naturally occur as metabolites of phytic acid. These compounds play crucial roles in physiological functions, including intracellular signalling, anti-inflammatory responses, and the prevention of diabetes-related complications.
Given the significance of inositol phosphates, reliable analytical methodologies are essential for their accurate determination. However, analysing these highly charged molecules is challenging due to their lack of characteristic absorption maxima in the ultraviolet or visible spectrum, necessitating post-column derivatization or other non-optical detection methods. Additionally, the presence of high levels of salts, ions, proteins, fats, and starch in food and feed matrices complicates sample preparation and analytical separation.
The new study:In reviewing existing analytical methods for separating inositol phosphate molecules, the authors concluded that individual isomer separation is only achievable through ion chromatography. For detection, they proposed ICP-OES, which eliminates the need for post-column derivatization and offers compound-independent calibration.
The method achieved the separation of 28 inositol phosphate isomers using an ion-chromatographic system with a CarboPac PA 100 analytical column (4 x 250 mm) and a nitric acid-water gradient within 33 minutes. This setup included a CarboPac PA100 guard column and an additional guard column to protect against food and feed matrices.
The ICP-OES spectrometer was purged with nitrogen to allow for sensitive detection of the phosphorus UV lines at 177.495 nm and 213.618 nm with axial viewing. The linear working range was 0.062-32 mg/L P, with limits of detection and quantification determined to be 63 µg/L P and 208 µg/L P, respectively.
The researchers also evaluated various acidic and alkaline extraction methods for food and feed samples, finding that the highest phytate extraction yield was achieved using 0.5 M HCl for 1 hour. Sample clean-up, necessary to remove matrix components such as carbohydrates, was performed using C18 SPE columns.
Using this developed methodology, the authors analysed nine samples, including grains, rice, beans, lentils, and pseudo-grains, for their phytate and inorganic phosphate content. The results demonstrated high reproducibility (under 4% for feed samples) and an 80% recovery rate for phytate. The authors concluded that their methodology is suitable for exploring enzymatic degradation pathways and analysing inositol phosphate in complex food and animal feed matrices.
 The original publication
The original publication

Corinna Henninger, Tobias Stadelmann, Daniel Heid, Katrin Ochsenreither, Thomas Eisele,
Ion chromatography coupled with optical emission spectrometry (IC-ICP-OES) methodology for the analysis of inositol phosphates in food and feed, Food Chemistry, 463 (2025) 141437.
DOI: 10.1016/j.foodchem.2024.141437
 Related studies
Related studies (newest first)

C. Henninger, B. Spangenberg, M. Schmidt, K. Ochsenreither, T. Eisele.
Enzyme-assisted HPTLC method for the simultaneous analysis of inositol phosphates and phosphate. JSFA Reports, 3/4 (2023) 170-179.
DOI: 10.1002/jsf2.109
G. Marolt, M. Kolar.
Analytical methods for determination of phytic acid and other inositol phosphates: A review. Molecules, 26/1 (2021) 174.
DOI: 10.3390/MOLECULES26010174
Q.H. Duong, K.D. Clark, K.G. Lapsley, R.B. Pegg.
Quantification of inositol phosphates in almond meal and almond brown skins by HPLC/ESI/MS. Food Chem., 229 (2017) 84–92.
DOI: 10.1016/J.FOODCHEM.2017.02.031
A. Rugova, M. Puschenreiter, J. Santner, L. Fischer, S. Neubauer, G. Koellensperger, S. Hann.
Speciation analysis of orthophosphate and myo-inositol hexakisphosphate in soil- and plant-related samples by high-performance ion chromatography combined with inductively coupled plasma mass spectrometry. J. Separat. Sci., 37/14 (2014) 1711–1719.
DOI: 10.1002/JSSC.201400026

Mieke Wintergerst,
Detection of inositol phosphates with HPLC-ICP-AES - Method development, Bachelor thesis, Uppsala University, 2013. available from
DiVA portal 
K. Blaabjerg, J. Hansen-Møller, H.D. Poulsen.
High-performance ion chromatography method for separation and quantification of inositol phosphates in diets and digesta. J. Chromatogr. B, 878/3–4 (2010) 347–354.
DOI: 10.1016/J.JCHROMB.2009.11.046
Z. Chen, W. He, M. Beer, M. Megharaj, R. Naidu.
Speciation of glyphosate, phosphate and aminomethylphosphonic acid in soil extracts by ion chromatography with inductively coupled plasma mass spectrometry with an octopole reaction system. Talanta, 78/3 (2009) 852–856.
DOI: 10.1016/J.TALANTA.2008.12.052
X. Liu, P.W. Villalta, S.J. Sturla.
Simultaneous determination of inositol and inositol phosphates in complex biological matrices: Quantitative ion-exchange chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom., 23/5 (2009) 705–712.
DOI: 10.1002/RCM.3923
L. Heighton, W.F. Schmidt, C.P. Rice, R.L. Siefert.
Electrospray ionization mass spectroscopy shows speciation of phytate to be pH dependent. J. Food Agricult. Environ., 6/2 (2008) 402–407.

Heidi De Brabandere, Niklas Forsgard, Lena Israelsson, Jean Petterson, Emil Rydin, Monica Waldebäck, Per J. R. Sjöberg,
Screening for Organic Phosphorus Compounds in Aquatic Sediments by Liquid Chromatography Coupled to ICP-AES and ESI-MS/MS, Anal. Chem., 80/17 (2008) 6689-6697.
DOI: 10.1021/ac8006335 
Q. Chen, B.W. Li.
Separation of phytic acid and other related inositol phosphates by high-performance ion chromatography and its applications. J. Chromatogr. A, 1018/1 (2003) 41–52.
DOI: 10.1016/J.CHROMA.2003.08.040
F. Grases, A. Llobera.
Determination of phytic acid in urine by ICP atomic emission spectrometry. Anal. Lett., 29/7 (1996) 1193–1199.
DOI: 10.1080/00032719608001468
 Related EVISA News (newest first):
Related EVISA News (newest first):
last time modified: September 7, 2025