A team of researchers from Germany and the US have developed a method based on ion-chromatography for the sensitive and selective determination of gadolinium-based contrast agents in surface waters. The method is implemented on an automated workstation coupled to an inductively coupled plasma mass spectrometer, providing fully automated analysis with high sample throughput, high selectivity and excellent detection power.
Background:Magnetic resonance imaging (MRI) is an indispensable method for clinical diagnostic, used for imaging inner organs. Gadolinium-based contrast agents (GBCAs) are used in 1 out of 3 MRI scans to improve the clarity and quality of images of the body's internal structures. Normally, a person of 75 kg body weight will receive a dose of about 1.2 g of Gadolinium.
The MRI agents are excreted nearly quantitatively from the patient’s body, unmetabolized and fast via the urine. Depending on the number and activity of clinical centres performing MRI examinations within the catchment area of a sewage treatment plant, the input of Gadolinium (Gd) into the public sewer is therefore relatively high. Hence, it can be estimated that in Europe alone, up to 19 tons of Gd are discharged into the environment every year, making GBCAs an emerging contaminant in aquatic systems.
As has been demonstrated in earlier research, the highly stable GBCAs are neither significantly retained nor degraded in standard waste water treatment plants, and are released into the aquatic environment. Since gadolinium is a "rare earth element", such pollution can enhance the natural background concentration of gadolinium in water markedly, which has been recognized by many scientists via the observation of an unnatural distribution pattern of the rare earth elements with an enhanced fraction of gadolinium called "gadolinium anomaly". However, such anomaly can also have biogeochemical causes resulting from the geological situation or the chemistry within the water body. In order to monitor the distribution of the GBCAs and their fate in the aquatic environment, therefore, a highly sensitive and selective speciation analysis is required. Since long-term ecotoxicological effects of GBCAs are mainly unknown, such speciation analysis is mandatory as a first step for gaining information about their environmental behaviour.
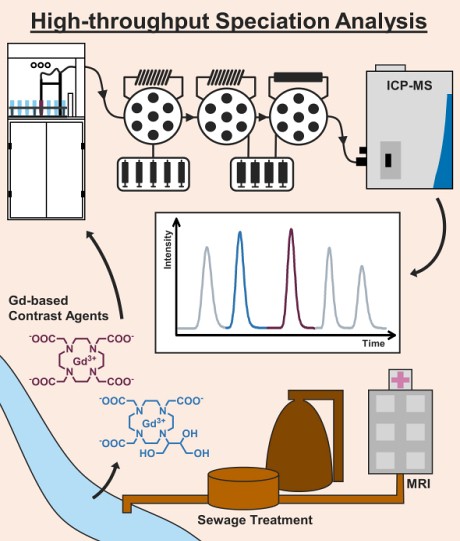
The new method:Working on this topic since 2006, the researchers from the University of Münster cooperated with the instrument manufacturer
Elemental Scientific (ESI) to fully automate the speciation analysis of GBCAs based on ion-chromatography coupled to inductively coupled plasma-mass spectrometry (ICP-MS). The developed method is able to determine the five complexes Gd-HP-DO3A, Gd-BT-DO3A, Gd-DOTA, Gd-DTPA, and Gd-BOPTA that are commonly administered in the European Union within a chromatographic run of less than 2 minutes.
The method is implemented on an automated workstation (
prepFast IC) for total metal analysis and syringe-driven chromatography. Furthermore, the use of an automated inline-dilution function allowed a fast-external calibration from single stock standards. Using an anion-exchange chromatography (IC) method for separation and a quadrupole ICP-MS for detection, species-specific detection limits between 11 and 19 pmol L-1 proved to be very competitive to previously published methods, that had to use special sample introduction systems (ultrasonic nebulizers, desolvation systems) in combination with high sensitivity sector-field ICP-MS.
The automated IC-ICP-MS method was applied to monitor GBCAs in a couple of surface waters collected from five different river and creek systems in the German state of North Rhine-Westphalia. All sampled surface waters are affected by the discharge of municipal WWTPs and are also relevant for the local drinking water supply. The complexes Gd-HP-DO3A, Gd-BT-DO3A, and Gd-DOTA, were detected and quantified. In addition, the occurrence of an unidentified Gd species was observed for one of the sampled river systems.
 The original publication
The original publication
Marcel Macke,
C. Derrick Quarles Jr, Michael Sperling,
Uwe Karst,
Fast and Automated Monitoring of Gadolinium-based Contrast Agents in Surface Waters, Water Res., 207 (2021) 117836. DOI: 10.1016/j.watres.2021.117836.
 Instrumentation used:
Instrumentation used:
 Thermo Scientific - iCAP-TQ ICP-MS
Thermo Scientific - iCAP-TQ ICP-MS
 Related studies
Related studies (newest first)

G. Trapasso, S. Chiesa, R. Freitas, E. Pereira,
What do we know about the ecotoxicological implications of the rare earth element gadolinium in aquatic ecosystems? Sci. Total Environ., 781 (2021) 146273.
DOI: 10.1016/j.scitotenv.2021.146273
M. Horstmann, R. Gonzalez de Vega, D.P. Bishop,
U. Karst, P.A. Doble,
D. Clases, Determination of gadolinium MRI contrast agents in fresh and oceanic waters of Australia employing micro-solid phase extraction, HILIC-ICP-MS and bandpass mass filtering. J. Anal. At. Spectrom., 36/4 (2021) 767-775.
DOI: 10.1039/d0ja00493f

S. Okabayashi, L. Kawane, N.Y. Mrabawani, T. Iwai, T. Narukawa, M. Tsuboi, K. Chiba,
Speciation analysis of Gadolinium-based contrast agents using aqueous eluent hydrophilic interaction liquid chromatography hyphenated with inductively coupled plasma-mass spectrometry. Talanta, 222 (2021) 121531.
DOI: 10.1016/j.talanta.2020.121531
U. Boester, T.R. Rüde,
Utilize gadolinium as environmental tracer for surface watergroundwater interaction in Karst. J. Contam. Hydrol., 235 (2020) 103710.
DOI: 10.1016/j.jconhyd.2020.103710 C.D. Quarles
C.D. Quarles, A.D. Toms, R. Smith, P. Sullivan, D. Bass, J. Leone,
Automated ICPMS method to measure bromine, chlorine, and iodine species and total metals content in drinking water. Talanta Open, 1 (2020) 100002.
DOI: 10.1016/j.talo.2020.100002

J. Rogowska, E. Olkowska, W. Ratajczyk, L. Wolska,
Gadolinium as a new emerging contaminant of aquatic environments. Environ. Toxicol. Chem., 37 (2018) 1523–1534.
DOI: 10.1002/etc.4116

H.S. Thomsen,
Are the increasing amounts of gadolinium in surface and tap water dangerous? Acta Radiol., 58 (2017) 259–263.
DOI: 10.1177/0284185116666419
M. Birka,
C.A. Wehe, O. Hachmöller,
M. Sperling,
U. Karst,
Tracing gadoliniumbased contrast agents from surface water to drinking water by means of speciation analysis. J. Chromatogr. A, 1440 (2016) 105–111.
DOI: 10.1016/j.chroma.2016.02.050
M. Birka, J. Roscher, M. Holtkamp,
M. Sperling,
U. Karst,
Investigating the stability of gadolinium based contrast agents towards UV radiation. Water Res., 91 (2016) 244–250.
DOI: 10.1016/j.watres.2016.01.012
U. Lindner, J. Lingott, S. Richter, W. Jiang,
N. Jakubowski, U. Panne,
Analysis of Gadolinium-based contrast agents in tap water with a new hydrophilic interaction chromatography (ZIC-cHILIC) hyphenated with inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem., 407 (2015) 2415–2422.
DOI: 10.1007/s00216-014-8368-5
M. Birka,
C.A. Wehe, L. Telgmann,
M. Sperling,
U. Karst,
Sensitive quantification of gadolinium-based magnetic resonance imaging contrast agents in surface waters using hydrophilic interaction liquid chromatography and inductively coupled plasma sector field mass spectrometry. J. Chromatogr. A, 1308 (2013) 125–131.
DOI: 10.1016/j.chroma.2013.08.017 L. Telgmann
L. Telgmann,
M. Sperling,
U. Karst,
Determination of gadolinium-based MRI contrast agents in biological and environmental samples: A review. Anal. Chim. Acta, 764 (2013) 1–16.
DOI: 10.1016/j.aca.2012.12.007
U. Lindner, J. Lingott, S. Richter,
N. Jakubowski, U. Panne,
Speciation of gadolinium in surface water samples and plants by hydrophilic interaction chromatography hyphenated with inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem., 405 (2013) 1865–1873.
DOI: 10.1007/s00216-012-6643-x L. Telgmann
L. Telgmann,
C.A. Wehe, M. Birka, J. Künnemeyer, S. Nowak,
M. Sperling,
U. Karst,
Speciation and isotope dilution analysis of gadolinium-based contrast agents in wastewater. Environ. Sci. Technol., 46 (2012) 11929–11936.
DOI: 10.1021/es301981z

S. Kulaksiz, M. Bau,
Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl. Geochem., 26 (2011) 1877–1885.
DOI: 10.1016/j.apgeochem.2011.06.011
D. Schwesig, A. Bergmann,
Use of anthropogenic gadolinium as a tracer for bank filtrate in drinking water wells. Water Sci. Technol. Water Supply, 11 (2011) 654–658.
DOI: 10.2166/ws.2011.097
P. Pfundstein, C. Martin, W. Schulz, R.K. Meike, A. Wille, T. Moritz, A. Steinbach, D. Flottman,
IC-ICP-MS Analysis of Gadolinium-Based MRI Contrast Agents. LC GC North Am., 29 (2011) 27–29.

C.S.K. Raju, A. Cossmer, H. Scharf, U. Panne, D. Lück,
Speciation of gadolinium based MRI contrast agents in environmental water samples using hydrophilic interaction chromatography hyphenated with inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom., 25 (2010) 55–61.
DOI: 10.1039/b919959d
J. Künnemeyer, L. Terborg,
B. Meermann, C. Brauckmann, I. Möller, A. Scheffer,
U. Karst,
Speciation analysis of gadolinium chelates in hospital effluents and wastewater treatment plant sewage by a novel HILIC/ICP-MS method. Environ. Sci. Technol., 43 (2009) 2884–2890.
DOI: 10.1021/es803278n
P. Möller, P. Dulski, M. Bau, A. Knappe, A. Pekdeger, C. Sommer-Von Jarmersted,
Anthropogenic gadolinium as a conservative tracer in hydrology. J. Geochemical Explor., 69–70 (2000) 409–414.
DOI: 10.1016/S0375-6742(00)00083-2
M. Bau, P. Dulski,
Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet. Sci. Lett., 143 (1996) 245–255.
DOI: 10.1016/0012-821X(96)00127-6
 Related EVISA News (Newest first)
Related EVISA News (Newest first)