A group of German researchers from Münster has used the combination of speciation analysis and native mass spectrometry as a tool to study diverse interactions of environmentally relevant organotin compounds with proteins.
Background:
Organotin compounds (OTCs) represent an essential class of organometallics in modern life. Due to their versatile characteristics, OTCs have been widely used in industry and agriculture since the 1950s, with an annual production surpassing 50 000 tons in the late 90s. They are used in marine activities and agriculture, as pesticides and fungicides; and in industry, for the manufacture of polyvinyl chloride (PVC) and silicone amongst other products where they act as catalysts.
Unfortunately, it was recognized only lately that some of those chemicals have the ability to mimic or antagonize the action of hormones, thus affecting the normal functioning of organisms leading to deleterious effects. Research on the complex toxicity of OTCs already started in the 1950s and showed that toxic effects depend on the target organism and the number and type of organic substituents on the central Sn(IV) atom (see Figure 1).
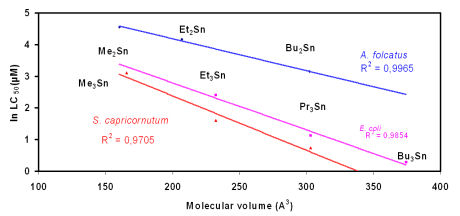
Figure 1: As has been
shown by Luedke et al., 1991, the toxicity of di- and
tri-organotin compounds (chlorides) is a
function of the molecular volume of the compound (and not of the inclusion of a "toxic element" tin in the compound !).
Studies on the interaction of OTCs with biological target molecules contributed to a further understanding of the different toxic effects leading to immunotoxicity, neurotoxicity and reproductive toxicity. As a result of such studies, several affected protein families and their OTC binding sites are known, for example, the nuclear receptor (NR) superfamily, the cytochrome P450 superfamily or different ATPases. While the trisubstituted OTCs (and especially tributyltin ) have been studied in some detail, the molecular targets and mechanisms of mono- and disubstituted OTCs (MOTCs and DOTCs) are still not fully understood today.
The new study:A group of researchers from the University of Münster developed a new approach for speciation analysis of organotins based on a mild size exclusion chromatographic (SEC) separation coupled to inductively coupled plasma mass spectrometry (ICP-MS) and native electrospray ionization mass spectrometry (ESI-MS). Using these complementary techniques, even weak interactions of OTCs with model proteins were investigated. One of the model proteins investigated was bovine ß-lactoglobulin A (LGA), a relatively small and well-established lipocalin-type protein with a ß-barrel motif and one reduced cysteine, which is solvent-accessible. Incubations of the protein with tributyltin (TBT) and triphenyltin (TPhT) were carried out under simulated physiological conditions. Several interactions between LGA and OTCs were observed, as the exemplary comparison of TBT and TPhT in Fig. 2 demonstrates.
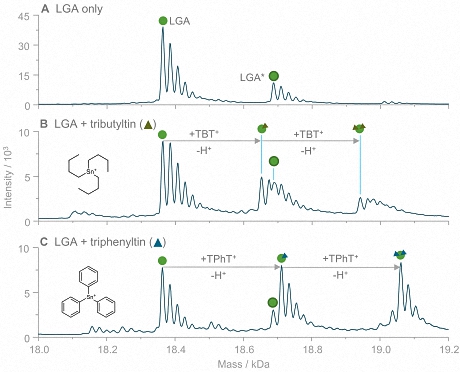
Figure 2: Adduct formation of lactoglobulin with tributyltin and triphenyltin. The shown deconvoluted mass spectra were acquired by direct injection ESI-MS analysis of LGA (A) and LGA incubated with TBT (B) and TPhT (C). For both TOTCs, signals corresponding to adduct species with stoichiometries of 1:1 and 1:2 (LGA to TOTC) were detected. The LGA-TPhT adducts revealed higher signal intensities (in relation to LGA) than LGA-TBT.
The authors further did some experiments to gain information about the stability of the adducts and to identify the binding site. It was shown that the adducts were sensitive to the concentration of organic solvents but even more sensitive to pH. At pH3, adduct formation of LGA with TPhT was fully inhibited. By further experiments with LGA including enzymatic digestion, and alkylation of reduced cysteine, the specific role of cysteine in binding trisubstituted OTCs (TOTCs) was investigated. The results of these experiments indicated that the reduced cysteine was mandatory for the binding of TPhT but not sufficient. In contrast to TOTCs, their natural di- and mono-substituted degradation products, such as dibutyltin, revealed to be less specific regarding their binding to several proteins. Furthermore, dibutyltin was observed to induce hydrolysis of the protein’s peptide backbone at a specific site.
The authors concluded that future studies on di- and mono-substituted OTCs are advisable in order to improve the knowledge about long-term toxic effects of these species.
 The original study:
The original study:
 Used techniques and instrumentation:
Used techniques and instrumentation:


 Related studies (newest first)
Related studies (newest first)

H.M. Wahba, M.J. Stevenson, A. Mansour, J. Sygusch, D.E. Wilcox and J.G. Omichinski,
Structural and Biochemical Characterization of Organotin and Organolead Compounds Binding to the Organomercurial Lyase MerB Provide New Insights into Its Mechanism of Carbon–Metal Bond Cleavage, J. Am. Chem. Soc., 139 (2017) 910–921.
DOI: 10.1021/jacs.6b11327
S. Harada, Y. Hiromori, S. Nakamura, K. Kawahara, S. Fukakusa, T. Maruno, M. Noda, S. Uchiyama, K. Fukui, J.-I. Nishikawa, H. Nagase, Y. Kobayashi, T. Yoshida, T. Ohkubo and T. Nakanishi,
Structural basis for PPARg transactivation by endocrine-disrupting organotin compounds, Sci. Rep., 5 (2015) 8520.
DOI: 10.1038/srep08520
P. Cardiano, G. Falcone, C. Foti, O. Giuffre, A. Napoli,
Binding ability of glutathione towards alkyltin(IV) compounds in aqueous solution, J. Inorg. Biochem., 129 (2013) 84–93. DOI:
10.1016/j.jinorgbio.2013.09.004
S. García-Carrillo, F.J. Aranda, A. Ortiz, J.A. Teruel,
Interaction of trialkyltin(IV) chlorides with sarcoplasmic reticulum calcium ATPase, Appl. Organomet. Chem., 26 (2012) 583–592. DOI:
10.1002/aoc.2903
A. Gianguzza, O. Giuffre, D. Piazzese, S. Sammartano,
Aqueous solution chemistry of alkyltin(IV) compounds for speciation studies in biological fluids and natural waters, Coord. Chem. Rev., 256 (2012) 222–239.
DOI: 10.1016/j.ccr.2011.06.027
S. Nesci, V. Ventrella, F. Trombetti, M. Pirini, A. Pagliarani,
Multi-site TBT binding skews the inhibition of oligomycin on the mitochondrial Mg-ATPase in Mytilus galloprovincialis, Biochimie, 93 (2011) 1157–1164. DOI:
10.1016/j.biochi.2011.04.008
Y. Hiromori, J.-I. Nishikawa, I. Yoshida, H. Nagase, T. Nakanishi,
Structure-dependent activation of peroxisome proliferator-activated receptor (PPAR) by organotin compounds, Chem.-Biol. Interact., 180 (2009) 238–244. DOI:
10.1016/j.cbi.2009.03.006
P. Cardiano, C. De Stefano, O. Giuffre, S. Sammartano,
Thermodynamic and spectroscopic study for the interaction of dimethyltin(IV) with L-cysteine in aqueous solution, Biophys. Chem., 133 (2008) 19–27. DOI:
10.1016/j.bpc.2007.11.005
B. A. Buck-Koehntop, F. Porcelli, J. L. Lewin, C. J. Cramer, G. Veglia,
Biological chemistry of organotin compounds: interactions and dealkylation by dithiols, J. Organomet. Chem., 691 (2006) 1748–1755.
DOI: 10.1016/j.jorganchem.2005.12.047
B. A. Buck, A. Mascioni, C. J. Cramer, G. Veglia,
Interactions of alkyltin salts with biological dithiols: dealkylation and induction of a regular b-turn structure in peptides, J. Am. Chem. Soc., 126 (2004) 14400–14410. DOI:
10.1021/ja046093s
K. Fent, B.R. Woodin, J.J. Stegeman,
Effects of triphenyltin and other organotins on hepatic monooxygenase system in fish, Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol., 121 (1998) 277–288.
DOI: 10.1016/S0742-8413(98)10048-8

E. Luedke, E. Lucero, G. Eng,
Molecular volume as a predictor of organotin biotoxicity, Main Group Metal Chemistry, 14 (1991) 59

K.R. Siebenlist, F. Taketa,
Organotin–protein interactions. Binding of triethyltin bromide to cat haemoglobin, Biochem. J., 233 (1986) 471–477. DOI:
10.1042/bj2330471

B.M. Elliott, W.N. Aldridge, J.W. Bridges,
Triethyltin Binding to Cat Haemoglobin. Evidence for Two Chemically Distinct Sites and a Role for both Histidine and Cysteine Residues, Biochem. J., 177 (1979) 461–470.
DOI: 10.1042/bj1770461
 Related EVISA News (newest first)
Related EVISA News (newest first)
last time modified: January 11, 2025