In classical toxicology, speciation of carbon is taken for granted and the carbon compounds responsible for toxicity are always described with the appropriate chemical nomenclature. By contrast, speciation of other elements is largely ignored and elements other than carbon are often condemned as toxic because of evidence relating toxicity to only a few of the chemical species in which they occur.
As an
example, the element arsenic is often taken as a synonym for poison, while the
arsenic compounds present in fish and other seafood are actually as harmless as
table salt:
| CHEMICAL
SPECIES | LD50 (mg/kg)
|
Arsenite
(As(III))
| 14
|
Arsenate
(As(V))
| 20
|
| Arsine
(AsH3) | 3 |
| Monomethylarsonic
Acid (MMA) | 700 - 1800 |
Dimethylarsinic
Acid (DMA)
| 700 - 2600 |
Arsenocholine
| > 10000 |
| Arsenobetaine | > 10000 |
LD50
rat: concentration leading to the death of 50 % of a rat population
Since the
physical, chemical and biological characteristics of a chemical substance
depend primarily on its molecular structure and not on one of its elemental
constituents, so does its toxicity.
As an
example let us discuss the toxicity of organotin compounds:
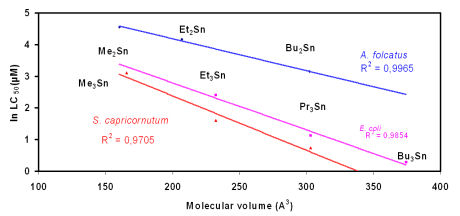
As has been
shown by Luedke et al., 1991, the toxicity of di- and
tri-organotin compounds (chlorides) depends on the target organism and is a
function of the molecular volume of the compound (and not of the inclusion of a "toxic element" tin in the compound !).
The
toxicity of often called “toxic trace
elements” depends on their speciation and concentration not only in a quantitative
way but also in a qualitative way.
Some examples:
- Cr(III) is
considered to be beneficial for the glucose metabolism while Cr(VI)
is cancerogen
- Inorganic
As(III) compounds are cancerogen while
Arsenobetaine is essential non-toxic
- Inorganic
tin compounds are discussed as being
essential for plants and some animals but
tributyltin (TBT) is an endocrine discuptor
The chemical species of a metal can effect its toxicity by influencing its
- absorption (or the physical availability for exposure - if the metal is tightly bound to in-absorbable material, it cannot be readily taken up, e.g. into the blood stream of the organism)
- distribution (the internal transport inside the organism to the tissue on which it has toxic effects - for example the crossing of the intestinal membrane or the blood-brain barrier)
- biotransformation (its accumulation, bio-modification, detoxification in – and excretion from – the tissues)
It is
therefore essential that toxicological studies should always consider the species
present rather than the elemental constituent in order to create meaningful data. With
respect to risk assessment and legislation it becomes more and more clear that failure to consider properly
chemical speciation of elements other than carbon can lead to poor use of our
resources. Laws and regulations based on simple elemental analysis may wrongly
condemn environmental media or products as toxic and prevent the use of
important resources.
When evaluating the toxicity of a target compound (species), the possibility of species conversion during the test period must be considered. Such conversion might be produced by reactions of the target compound with components of the studied system (e.g. cell culture media) such as redox reactions, hydrolysis, adduct formation or complexation, leading to declining concentrations of the target compound and appearance of new species. For such reasons, toxicity test should be accompanied by analytical testing verifying that the species present is the target compound and the concentration level being present during the exposure time is the target exposure level. If that is not the case, time weighted exposure levels can be used instead of concentration levels.
 Related EVISA Resources
Related EVISA Resources Link Database: Toxicity of Elemental Species
Link Database: Toxicity of Elemental Species Further Reading
Further Reading
 H.E. Allen, R.H. Hall, T.D. Brisbin, Metal speciation. Effect on aquatic toxicity, Environ. Sci. Technol., 14/4 (1980) 441-443. DOI: 10.1021/es60164a002
H.E. Allen, R.H. Hall, T.D. Brisbin, Metal speciation. Effect on aquatic toxicity, Environ. Sci. Technol., 14/4 (1980) 441-443. DOI: 10.1021/es60164a002
 M.L. Freedman, P.M. Cunningham, J.E. Schindler, M.J. Zimmerman, Effect of lead speciation on toxicity, Bull. Environ. Contam. Toxicol., 25/1 (1980) 389-393. DOI: 10.1007/BF01985543
M.L. Freedman, P.M. Cunningham, J.E. Schindler, M.J. Zimmerman, Effect of lead speciation on toxicity, Bull. Environ. Contam. Toxicol., 25/1 (1980) 389-393. DOI: 10.1007/BF01985543
 G.M.P. Morrison, G.E. Batley, T.M. Florence, Metal speciation and toxicity, Chem. Br., 25 (1989) 791-796. available from ResearchGate
G.M.P. Morrison, G.E. Batley, T.M. Florence, Metal speciation and toxicity, Chem. Br., 25 (1989) 791-796. available from ResearchGate
 T. Wolf, R. Kasemann, H. Ottenwälder, Molecular interaction of different chromium species with nucleotides and nucleic acids, Carcinogenesis, 10/4 (1989) 655-659. DOI: 10.1093/carcin/10.4.655
T. Wolf, R. Kasemann, H. Ottenwälder, Molecular interaction of different chromium species with nucleotides and nucleic acids, Carcinogenesis, 10/4 (1989) 655-659. DOI: 10.1093/carcin/10.4.655
 K.A. Biedermann, J.R. Landolph, Role of valency state and solubility of chromium compounds on induction of cytotoxicity, mutagenesis, and anchorage independence in diploid human fibroplasts, Cancer Res., 50/24 (1990) 7835-7842.
K.A. Biedermann, J.R. Landolph, Role of valency state and solubility of chromium compounds on induction of cytotoxicity, mutagenesis, and anchorage independence in diploid human fibroplasts, Cancer Res., 50/24 (1990) 7835-7842.

E. Luedke, E. Lucero, G. Eng,
Molecular volume as a predictor of organotin biotoxicity, Main Group Metal Chemistry, 14 (1991) 59

T. Kaise, S. Fukui,
The chemical form and acute toxicity of arsenic compounds in marine organisms, Appl. Organomet. Chem., 6/2 (1992) 155-160.
DOI: 10.1002/aoc.590060208
S.B. Jonnalagadda, P.V. Rao,
Toxicity, bioavailability and metal speciation, Comp. Biochem. Physiol. C, 106/3 (1993) 585-595.
doi: 10.1016/0742-8413(93)90215-7
Guy Berthon,
Chemical speciation studies in relation to aluminium metabolism and toxicity, Coord. Chem. Rev., 149 (1996) 241-280.
DOI: 10.1016/S0010-8545(96)90030-2

D.M. Templeton,
Trace element speciation in toxicology and clinical sciences, Analusis, 26/6 (1998) M68-M71.
DOI: 10.1051/analusis:199826060068
S.D. Kim, H. Ma, H.E. Allen, D.K. Cha,
Influence of dissolved organic matter on the toxicity of copper to Ceriodaphnia dubia: effect of complexation kinetics, Environ. Toxicol. Chem., 18 (1999) 2433-2437.
DOI: 10.1002/etc.5620181108
Evert Nieboer, Glenn G. Fletcher, Yngvar Thomassen,
Relevance of reactivity determinants to exposure assessment and biological monitoring of the elements, J. Environ. Monit., 1 (1999) 1–14.
DOI: 10.1039/a808849g
S.H. Reaney, C.L. Kwik-Uribe, D.R. Smith,
Manganese oxidation state and its implications for toxicity, Chem. Res. Toxicol., 15 (2002) 1119–1126.
DOI: 10.1021/tx025525e
D. Bagchi, S.J. Stohs, B.W. Downs, M. Bagchi, H.G. Preuss,
Cytotoxicity and oxidative mechanisms of different forms of chromium, Toxicol., 180/1 (2002) 5-22.
DOI: 10.1016/S0300-483X(02)00378-5
L. Normandin, L. Ann Beaupre, F. Salehi, A. St -Pierre, G. Kennedy, D. Mergler, R.F. Butterworth, S. Philippe, J. Zayed,
Manganese distribution in the brain and neurobehavioral changes following inhalation exposure of rats to three chemical forms of manganese, Neurotoxicology, 25 (2004) 433– 441.
DOI: 10.1016/j.neuro.2003.10.001
J.H. Duffus,
Chemical speciation terminology: chromium chemistry and cancer, Mineral. Mag. (London), 69/5 (2005) 557-562.
DOI: 10.1180/0026461056950270
K.F. Akter, G. Owens, D.E. Davey, R. Naidu,
Arsenic speciation and toxicity in biological systems, Rev. Environ. Contam. Toxicol., 184 (2005) 97-149.
DOI: 10.1007/0-387-27565-7_3
P. Apostoli, R. Cornelis, J. Duffus, P. Hoet, D. Lison, D. Templeton,
Elemental Speciation in Human Health Risk Assessment, WHO,
Environmental Health Criteria #234 (2006)
Richard J. Reeder, Martin A. A. Schoonen, Antonio Lanzirotti,
Metal Speciation and Its Role in Bioaccessibility and Bioavailability, Rev. Mineral. Geochem., 64/1 (2006) 59-113.
DOI: 10.2138/rmg.2006.64.3
B. Michalke, S. Halbach, V. Nischwitz,
Metal speciation related to neurotoxicity in humans, J. Environ. Monit., 11 (2009) 939-954.
DOI: 10.1039/b817817h
Y. Ogra,
Toxicometallomics for Research on the Toxicology of Exotic Metalloids Based on Speciation Studies, Anal. Sci., 25/10 (2009) 1189-1195.
DOI: 10.2116/analsci.25.1189
L. Lévesque, C.A. Mizzen, D.R. McLachlan, P.E. Fraser,
Ligand specific effects on aluminum incorporation and toxicity in neurons and astrocytes, Brain Res., 877 (2009) 191-202.
DOI: 10.1016/S0006-8993(00)02637-8
T.L. Pan, P.W. Wang, S.A. Al-Suiwayeh, C.C. Chen, J.Y. Fang,
Skin toxicology of lead species evaluated by their permeability and proteomic profiles: A comparison of organic and inorganic lead, Toxixol. Lett., 197/1 (2010) 19-28.
DOI: 10.1016/j.toxlet.2010.04.019
D.A.L. Vignati, J. Dominik, M.L. Beye, M. Pettine, B.J.D. Ferrari,
Chromium(VI) is more toxic than chromium(III) to freshwater algae: A paradigm to revise ?, Ecotoxicol. Environ. Safety, 73/5 (2010) 743-749.
DOI: 10.1016/j.ecoenv.2010.01.011
N. Strigul, A. Koutsospyros, C. Christodoulatos,
Tungsten speciation and toxicity: Acute toxicity of mono- and poly-tungstate to fish, Ecotoxicol. Environ. Safety, 73/2 (2010) 164-171.
DOI: 10.1016/j.ecoenv.2010.08.016
G. Papathanasiou, K.N. White, R. Walton, S. Boult,
Toxicity of aluminium in natural waters controlled by typew rather than quantity of natural organic matter, Sci. Total. Environ., 409/24 (2011) 5277-5283.
DOI: 10.1016/j.scitotenv.2011.08.064
Malgorzata Korbas, Tracy C. MacDonald, Ingrid J. Pickering, Graham N. George, Patrick H. Krone,
Chemical
Form Matters: Differential Accumulation of Mercury Following Inorganic
and Organic Mercury Exposures in Zebrafish Larvae, ACS Chem. Biol., 7/2 (2012) 411–420.
DOI: 10.1021/cb200287c
Douglas M. Templeton,
Speciation in Metal Toxicity and Metal-Based Therapeutics, Toxics, 3/2 (2015), 170-186;
DOI:10.3390/toxics3020170

Daniela B. Friedman, Christopher Toumey, Dwayne E. Porter, Jie Hong, Geoffrey I. Scott, Jamie R. Lead,
Communicating with the public about environmental health risks: A community-engaged approach to dialogue about metal speciation and toxicity, Environ. Int., 74 (2015) 9-12.
DOI: 10.1016/j.envint.2014.09.015

Hani A. Alhadrami, Lenka Mbadugha, Graeme I. Paton,
Hazard and risk assessment of human exposure to toxic metals using in vitro digestion assay, Chem. Speciation Bioavail., 28/1-4 (2016) 78-87.
doi: 10.1080/09542299.2016.1180961

G. Genchi, G. Lauria, A. Catalano, A. Carocci, M.S. Sinicropi,
The Double Face of Metals: The Intriguing Case of Chromium, Appl. Sci., 11/2 (2021) 638.
DOI: 10.3390/app11020638

Jian Liu, Yuting Wen, Yucong Mo, Weizhen Liu, Xiliang Yan, Hongyu Zhou, Bing Yan,
Chemical speciation determines combined cytotoxicity: Examples of biochar and arsenic/chromium, J. Hazard. Mater., 448 (2023) 130855.
DOI: 10.1016/j.jhazmat.2023.130855

E. Tipping, S. Lofts, A. Stockdale,
WHAM-FTOXß - An aquatic toxicity model based on intrinsic metal toxic potency and intrinsic species sensitivity, Aquat. Toxicol., 258 (2023) #106503.
DOI: 10.1016/j.aquatox.2023.106503
 Related EVISA News
Related EVISA News (newest first)
 August 16, 2016: Toxicity and bioavailability of different selenium metabolites
August 16, 2016: Toxicity and bioavailability of different selenium metabolites April 17, 2015: Effect of dissolved humic acid on the bioavailability of lead from contaminated soil
April 17, 2015: Effect of dissolved humic acid on the bioavailability of lead from contaminated soil October 29, 2014: Side effects of cisplatin chemotherpy: Platinum speciation matters
October 29, 2014: Side effects of cisplatin chemotherpy: Platinum speciation matters  August 18, 2014: New research indicates that chromium (III) is even more genotoxic than chromium (VI)
August 18, 2014: New research indicates that chromium (III) is even more genotoxic than chromium (VI)  April 29, 2014: Arsenic’s Toxicity: Microbiome Alterations in the Mouse Gut
April 29, 2014: Arsenic’s Toxicity: Microbiome Alterations in the Mouse Gut  January 31, 2014: The Role of Phytochelatins for Metal Detoxification in Animals
January 31, 2014: The Role of Phytochelatins for Metal Detoxification in Animals  December 29, 2013: A new study finds: Inorganic mercury stays in the brain for years if not decades
December 29, 2013: A new study finds: Inorganic mercury stays in the brain for years if not decades  September 12, 2013: Scientists reveal how organic mercury can interfere with vision
September 12, 2013: Scientists reveal how organic mercury can interfere with vision  October 12, 2012: Prenatal mercury intake linked to ADHD
October 12, 2012: Prenatal mercury intake linked to ADHD  June 19, 2012: Vaccine ingredient causes brain damage; some nutrients prevent it
June 19, 2012: Vaccine ingredient causes brain damage; some nutrients prevent it  February 15, 2012: Cadmium Exposure Could Be Damaging Children’s Health more than Lead
February 15, 2012: Cadmium Exposure Could Be Damaging Children’s Health more than Lead  May 26, 2011: Oral ingestion of hexavalent chromium through drinking water and cancer mortality
May 26, 2011: Oral ingestion of hexavalent chromium through drinking water and cancer mortality  August 2, 2010: Gut bacteria transform inorganic arsenate leading to more toxic arsenic species
August 2, 2010: Gut bacteria transform inorganic arsenate leading to more toxic arsenic species  June 12, 2010: Chromium(VI) much more toxic than chromium(III): At least for
freshwater algae a paradigm to revise?
June 12, 2010: Chromium(VI) much more toxic than chromium(III): At least for
freshwater algae a paradigm to revise?  January 14, 2010: Form of Mercury in Older Dental Fillings Unlikely to be Toxic
January 14, 2010: Form of Mercury in Older Dental Fillings Unlikely to be Toxic  November 20, 2006: Metal species and Alzheimer disease
November 20, 2006: Metal species and Alzheimer diseaselast time modified: March 15, 2025