A spanish-german research team now found a way to detect trace amounts of methylmercury by a simple and quick strip-test.
Background:Mercury is entering our environment both via natural sources (weathering of rock, volcanism) and anthropogenic sources (coal burning, waste combustion, cement production, chlor-alkali electrolysis etc.). Once in the environment, mercury can be transformed by micro-organisms into methylmercury, which due to its high bioavailability is entering the food chain and gets further concentrated by bioaccumulation and biomagnification. Mercury is a toxic element and especially the nervous system is very sensitive to all forms of mercury. Methylmercury and metallic mercury vapours are more harmful than other forms, because more mercury in these forms reaches the brain. Effects on brain functioning may result in irritability, shyness, tremors, changes in vision or hearing, and memory problems. Organomercury compounds, such as methylmercury are the strongest neurotoxins, since these compounds are able to cross the blood-brain barrier easily. Further EPA has determined that mercuric chloride and methylmercury are possible human carcinogens.
The toxicity difference of the mercury compounds is the driving force to differentiate between the mercury species when analysing the concentrations of mercury in the environment or biological systems. Mercury speciation analysis normally requires the use of sophisticated analytical instrumentation consisting of a hyphenated system combining a separation module with a very sensitive detection module such as GC-MIP-AES, GC-ICP-MS, GC-AFS or LC-ICP-MS, to name only few. Besides sophisticated instrumentation, speciation analysis requires well trained experts and a laboratory infrastructure.
The new study:A spanish-german research team now found a way to detect methylmercury by a simple enough reaction that could be performed on-site. The researchers reported their findings in the October issue of
Angewandte Chemie (volume 121/45). The team around
Ramón Martínez-Máñez from the
Polytechnic University of Valencia and Knut Rurack
from the "
Bundesanstalt für Materialforschung und -prüfung" (BAM) make use of the strong affinity of methylmercury for sulphur.
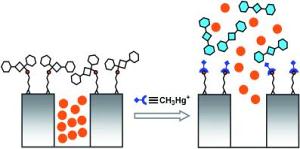
The researchers filled a dye into the cavities of a porous mineral and encapsulated the pores with a organic compound having sulphur atoms.
When mercury species interact with this test system, mercury binds to the sulphur atoms thereby cleaving the organic compound from the porous mineral. The release of the organic compound is opening the pores and discharging the dye. The speciality of this approach is the amplifying effect resulting from the effect that few mercury atoms are enough to release a high amount of dye molecules residing in a single cavity. In this way trace amounts of mercury can be detected by a simple optical detection method. Further, the test system can be embedded into a hydrophobic carrier that hinders inorganic mercury to reach the system. In this way the test system can be made selective for organo-mercury compounds.
The researchers envisage a test strip that dipped into a liquid sample would indicate the presence of organomercury compounds.
Source: (in part adapted and translated from) idw
 The original study
The original study
Estela Climent, M. Dolores Marcos, Ramón Martínez-Máñez, Félix Sancenón, Juan Soto, Knut Rurack, Pedro Amorós,
The Determination of Methylmercury in Real Samples Using Organically Capped Mesoporous Inorganic Materials Capable of Signal Amplification, Angew. Chemie, 121/45 (2009) 8671-8674.
DOI: 10.1002/ange.200904243 Related EVISA Resources
Related EVISA Resources Brief summary: GC-ICP-MS: A very sensitive hyphenated system for speciation analysis
Brief summary: GC-ICP-MS: A very sensitive hyphenated system for speciation analysis  Related EVISA News (newest first)
Related EVISA News (newest first)
 April 2, 2013: JRC validates simple method to monitor methylmercury in fish
April 2, 2013: JRC validates simple method to monitor methylmercury in fish September 10, 2009: Speciation Analysis - Striving for Quality
September 10, 2009: Speciation Analysis - Striving for Quality  November 8, 2006: Double spiking species-specific isotope dilution result calculations simplified
November 8, 2006: Double spiking species-specific isotope dilution result calculations simplified last time modified: May 21, 2024