Researchers from Brazil found that side effects of cisplatin chemotherapy appeared to be dependent on the brand of the drug.
Background:
After their discovery in the 1960s and since first clinical tests in 1974, platinum based cytostatic agents are frequently and effectively used in the fight against different types of tumors.
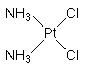
Cisplatin, a neutral and planar complex with two cis-chlorido and two ammine ligands binding at the central platinum atom (cis-diamminedichloroplatinum(II), CDDP), is the oldest of the platinum-based chemotherapeutics and was introduced in 1979. Cisplatin is still broadly applied to treat solid tumors such as testicular and ovarian cancer, bladder and lung tumors as well as the high malignant brain tumor called medulloblastoma. However, cisplatin has also many serious side effects such as nephrotoxicity, ototoxicity, neurotoxicity and emetogenicity.
Further, cisplatin can cause hypersensitivity reactions in 1–5% of the population when used as a single agent and 1–20% when used in combination with other antineoplastic agents.
Hypersensitivity to a chemotherapeutic agent is defined as an unforeseen reaction whose signs and symptoms cannot be explained by the known toxicity of the drug. Industrial complex platinum salts have been reported to cause hypersensitivity among exposured workers since 1945. Hypersensitivity reactions in patients receiving cisplatin were first described in the 1970s in patients who had been retreated with the drug. Since then, all platinum agents have been associated with such reactions. Symptoms include skin reactions, difficult breathing, coughing, chest tightness and anaphylactic shock. The exact mechanism of platinum allergy remains unclear although multiple studies have tried to explain the underlying pathophysiology, suggesting that various immunological and nonimmunological mechanisms are involved.
Researchers from Sao Paulo (Brazil) observed cases of hypersensitivity that appeared to be associated with the brand of cisplatin used. In order to study such hypothesis they compared two different brands of cisplatin in relation to type I hypersensitivity reactions. Brand A was used in a tertiary care teaching hospital until 2012, and use of brand B started from January 2013, when the first hypersensitivity cases were observed. Patients were categorized based on symptom. Cisplatin of both brands was analysed by high-performance liquid chromatography (HPLC) and high-resolution electrospray ionization mass spectrometry (ESI-(+)-MS) and characterized according to US Pharmacopeia. Both brands of cisplatin were in accordance with the US Pharmacopeia parameters, and there was no significant difference in the total platinum levels between the two brands when analysed by HPLC.
However, while there were no cases of hypersensitivity associated with the use of cisplatin brand A, four of 127 outpatients that used cisplatin brand B were affected. For improved drug characterization mass spectrometry was performed using a
LTQ-XL Orbitrap Discovery (Thermo Scientific, Bremen, Germany) for platinum speciation analysis. Compound identification was performed using both high-resolution and MS/MS reactions using collision-induced dissociation (CID) with helium as the buffer gas and collision energies ranging from 30 to 50 eV.
The relationship between cisplatin and total platinum-containing species was measured using the relative intensity as the main parameter. The observed molecules were grouped as the sum of whole cisplatin content vs. whole platinum-containing species. For hydrolysis products, the signals of [Pt - OH]
2+ and [Pt - Cl]
2+ were considered; for the parent molecules, the signals of [M + H]
+ and [M + Na]
+ were chosen. Considering the hydrolysed/intact ratios, data show that samples from brand B had a 2.7-fold higher concentration of hydrolytic products than brand A.
The researchers argue that the hydrolysed products of cisplatin are more toxic than the molecular form and are more kinetically active than the intact drug. Therefore it seems plausible that the higher concentration of the free form in brand B may be the cause of the observed hypersensitivity-induced reaction. The suggested mechanism to explain such enhanced side effects is based on the fact that the free-form platinum binds to proteins, forming an allergenic complex. Brand B drug had more free platinum, as seen from the MS results, and potentially forms more allergenic complexes, inducing type I hypersensitivity more frequently.
The researchers conclude that the enhanced characterization of the cisplatin formulation with respect to hydrolysed species was not part of the standard U.S. Pharmacopeia test to ensure the quality control of the drug, but should be considered by drug regulatory agencies and manufacturers to improve patients’ safety.
 The new study
The new study:

E.C. Pincinato, M.B. Visacri, C.M. de Souza, B.T. Tuan, G.B. Ferrari, D.N. de Oliveira, C.R. Barbosa, R.F. Rodrigues, S. Granja, R.F.L. Ambrosio, R.R. Catharino, P.C.P. Rosa, C.S.P. Lima, P.G. Mazzola and P. Moriel,
Impact of drug formulation and free platinum/cisplatin ratio on hypersensitivity reactions to cisplatin: formulation matters, J. Clin. Pharm. Ther., 40 (2015) 41-47.
doi: 10.1111/jcpt.12220 Related studies (newest first):
Related studies (newest first):

C. Brauckmann,
C.A. Wehe, M. Kieshauer, C. Lanvers-Kaminsky,
M. Sperling,
U. Karst,
The interaction of platinum-based drugs with native biologically relevant proteins, Anal Bioanal Chem (2013) 405:1855–1864.
doi: 10.1007/s00216-012-6410-z Björn Meermann
Björn Meermann,
Michael Sperling,
Hyphenated techniques as tools for speciation analysis of metal-based pharmaceuticals: developments and applications, Anal. Bioanal. Chem. (2012) 403 (2012) 1501–1522.
doi: 10.1007/s00216-012-5915-9
Susanne Nussbaumer, Sandrine Fleury-Souverain, Julie Schappler, Serge Rudaz, Jean-Luc Veuthey, Pascal Bonnabry,
Quality control of pharmaceutical formulations containing cisplatin, carboplatin, and oxaliplatin by micellar and microemulsion electrokinetic chromatography (MEKC, MEEKC), J. Pharma. Biomed. Anal., 55 (2011) 253–258.
doi: 10.1016/j.jpba.2011.01.029
N. Makrilia, E. Syrigou, I. Kaklamanos, L. Manolopoulos, M.W. Saif,
Hypersensitivity reactions associated with platinum antineoplastic
agents: a systematic review, Met-Based Drugs, 2010 (2010) 11.
doi: 10.1155/2010/207084 Bernhard Michalke, Platinum speciation used for elucidating activation or inhibition of Pt-containing anti-cancerdrugs
Bernhard Michalke, Platinum speciation used for elucidating activation or inhibition of Pt-containing anti-cancerdrugs, J. Trace Elements Med. Biol., 24 (2010)69–77.
doi: 10.1016/j.jtemb.2010.01.006
M.E. Bosch, A.J.R. Sánchez, F.S. Rojas, C.B. Ojeda,
Analytical methodologies for the determination of cisplatin, J. Pharmacol. Biomed. Anal., 47 (2008) 451–459.
doi: 10.1016/j.jpba.2008.01.047
C.A. Rabik, M.E. Dolan,
Molecular mechanisms of resistance and toxicity
associated with platinating agents, Cancer Treat. Rev., 33 (2007) 9–23.
doi: 10.1016/j.ctrv.2006.09.006
S.J. Lippard,
Chemistry and molecular biology of platinum anticancer drugs, Pure Appl. Chem., 59 (1987) 731–742.
doi: 10.1351/pac198759060731
R.B.
Weiss, S. Bruno,
Hypersensitivity reactions to cancer chemotherapeutic
agents, Ann. Int. Med., 94 (1981) 66–72.
doi: 10.7326/0003-4819-94-1-66
B. Rosenberg, L. Van Camp, J.E. Trosko, V.H. Mansour,
Platinum compounds: a new class of potent antitumour agents, Nature, 222 (1969) 385–386.
doi: 10.1038/222385a0
 Related EVISA Resources
Related EVISA Resources
 Brief summary: ESI-MS: The tool for the identification of species
Brief summary: ESI-MS: The tool for the identification of species Brief summary: Speciation analysis for the study of metallodrugs and their biomolecular interactions
Brief summary: Speciation analysis for the study of metallodrugs and their biomolecular interactions
 Instrument Database: LTQ Orbitrap XL Mass spectrometer
Instrument Database: LTQ Orbitrap XL Mass spectrometer Material Database: Platinum standards for drug testing
Material Database: Platinum standards for drug testing Link database: Toxicity of platinum compounds
Link database: Toxicity of platinum compounds  Link page: All about mass spectrometry
Link page: All about mass spectrometry  Link page: All about platinum
Link page: All about platinum Glossary: Metallodrugs
Glossary: Metallodrugs
 Journal Database: Journals related to Medicinal Chemistry
Journal Database: Journals related to Medicinal Chemistry  Journal Database: Journals related to Pharmacology and Pharmacy
Journal Database: Journals related to Pharmacology and Pharmacy
 Journal Database: Journals related to Oncology
Journal Database: Journals related to Oncology
 Journal Database: Journals related to Metallomics
Journal Database: Journals related to Metallomics
 Link Database: Research groups working on metallodrugs
Link Database: Research groups working on metallodrugs
 Directory of scientists: Researchers working on metallodrugs
Directory of scientists: Researchers working on metallodrugs
 Link page: All about Pharmacy and Pharmaceutical Sciences
Link page: All about Pharmacy and Pharmaceutical Sciences
 Related EVISA News (newest first)
Related EVISA News (newest first)
last time modified: January 16, 2025