Chinese researchers have developed a method for the determination of platinum chloride complexes as impurities of platinum-based drugs.
Background:Platinum-based drugs are widely used in chemotherapy against a broad range of cancer types. Cisplatin (1978), carboplatin (1989) and oxaliplatin (2002) are three members of the platinum anticancer drug family approved in many counties. These compounds have poor oral bioavailability and are therefore administered by injection, a method in which serious safety problems can be caused by impurities.
Impurities can be introduced during the synthesis process of materials by reagents, intermediates, by-products, and isomers. In general, potassium chloroplatinate (K2PtCl4) or potassium hexachloroplatinate (K2PtCl6) are raw materials for the synthesis of platinum-based drugs. Therefore, the residues of K2PtCl4 and K2PtCl6 in the final platinum-based drugs should be strictly controlled as part of the quality control. While the rule makers in the different countries have established limits for individual impurities or total impurities, they do not provide any methods for the quantification of K2PtCl4 and K2PtCl6. The European Pharmacopoeia (EP 8.0) has specified that the content of individual impurities in platinum-based drugs should not exceed 0.1%.
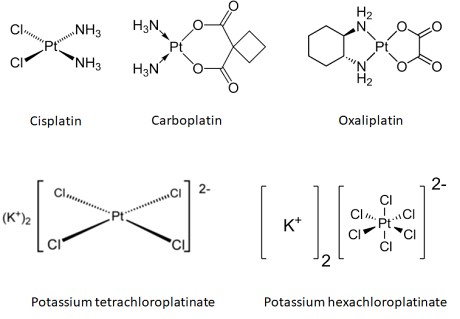
Figure: Structures of the platinum compounds discussed here
The new study:A group of Chinese researchers aimed at developing such method which can provide a reference basis for the scientific evaluation of the safety of platinum-based drugs. Typically, Pt speciation analysis is performed by using a separation method such as GC, CE, HILIC or HPLC coupled with a sensitive detection technique such as ICP-MS. The hyphenated technique HPLC-ICP-MS is particularly attractive for liquid samples, due the ease of sample preparation, the simple interface and the many separation modes available.
One of the challenges to quantify the Pt-impurities in Pt-based drugs is the low concentration of the impurities that has to be detected in presence of very high concentration of the Pt-drug contaminating and saturating the detection system. The authors therefore used an on-line switching technology meant to bypass the Pt-drug elution peak to waste instead of feeding it to the ICP-MS. In order to program the switching device, the authors investigated the elution sequence of the different compounds by injecting a mixed standard. Different columns were tested and the Hamilton PRP-X100 analytical column was selected as the optimum. In order to avoid any long time stability problems by introducing high concentrations of buffer systems, a low concentration of HCl solution (2 mmol/L) was used as mobile phase for isocratic elution. Using these HPLC parameters the 5 Pt species were separated in less than 5 min. in the order of cisplatin (0.2-0.5 min), oxaliplatin (0.6-0.8 Min), K2PtCl6 (1.5-2.0 min), K2PtCl4 (2.1-2.7 min) and carboplatin (3.4-4.2 min). Therefore, in order to bypass the Pt-drugs, the solution flowing from the column was discharged to waste in the range of 0.2-1 min and 3-5min. This allowed for the sensitive detection of the two impurities, with limits of quantitation of 0.7 µg/L for K2PtCl6 and 1.0 µg/l for K2PtCl4. The accuracy of the method was checked by spiking real samples with the target analytes. Spike recoveries between 94% and 103% demonstrated satisfactory accuracy.
 The original study
The original study

Z.Z. Yao, B.R. Li, C. Li, B.H. Wang, M. Zhao and Z.H. Ma,
Ultra-sensitive speciation analysis of inorganic platinum-chloride complexes in platinum-based drugs by HPLC-ICP-MS, J. Anal. At. Spectrom., 37/8 (2022) 1652-1657.
DOI: 10.1039/D2JA00126H
 Instrumentation used:
Instrumentation used:
 PerkinElmer - Flexar HPLC system
PerkinElmer - Flexar HPLC system PerkinElmer - NexION 350 ICP-MS
PerkinElmer - NexION 350 ICP-MS
 Related studies (newest first)
Related studies (newest first)
B.R. Li, C Li, J. Liu, Z.Y. He, F. Zhao, Z.H. Ma.
Measurement of trace potassium hexachloroplatinate concentrations in carboplatin by HPLC-ICP-MS with valve switching. J. Anal. At. Spectrom., 36 (2021) 2467-2472.
DOI: 10.1039/d1ja00207d

G. A. Zachariadis, O. E. Misopoulou.
Determination of cisplatin and carboplatin anticancer drugs by non-suppressed ion chromatography with an inductively coupled plasma atomic emission detector. Anal. Lett., 51 (2018) 1060-1070.
DOI: 10.1080/00032719.2017.1366498
J. Vidmar, A. Martinčič,
R. Milačič,
J. Scancar.
Speciation of cisplatin in environmental water samples by hydrophilic interaction liquid chromatography coupled to inductively coupled plasma mass spectrometry. Talanta, 138 (2015) 1-7.
DOI: 10.1016/j.talanta.2015.02.008
A. Martinčič, M. Cemazar, G. Sersa, V. Kovač,
R. Milačič,
J. Ščančar.
A novel method for speciation of Pt in human serum incubated with cisplatin, oxaliplatin and carboplatin by conjoint liquid chromatography on monolithic disks with UV and ICP-MS detection. Talanta, 116 (2013) 141-148.
DOI: 10.1016/j.talanta.2013.05.016
T. Falta, P. Heffeter, A. Mohamed, W. Berger,
S. Hann,
G. Koellensperger.
Quantitative determination of intact free cisplatin in cell models by LC-ICP-MS. J. Anal. At. Spectrom., 26 (2011) 109-115.
DOI: 10.1039/c0ja00047g
T. Falta,
G. Koellensperger, A. Standler, W. Buchberger, R.M. Mader,
S.T. Hann.
Quantification of cisplatin, carboplatin and oxaliplatin in spiked human plasma samples by ICP-SFMS and hydrophilic interaction liquid chromatography (HILIC) combined with ICP-MS detection. J. Anal. At. Spectrom., 24 (2009) 1336-1342.
DOI: 10.1039/b907011g

Y. Nygren, P. Hemström, C. Åstot.
Hydrophilic interaction liquid chromatography (HILIC) coupled to inductively coupled plasma mass spectrometry (ICPMS) utilizing a mobile phase with a low-volatile organic modifier for the determination of cisplatin, and its monohydrolyzed metabolite. J. Anal. At. Spectrom., 23 (2008) 948-954.
DOI: 10.1039/b716093c

D.N. Bell, J.J. Liu, M.D. Tingle, M.J. McKeage.
Specific determination of intact cisplatin and monohydrated cisplatin in human plasma and culture medium ultrafiltrates using HPLC on-line with inductively coupled plasma mass spectrometry. J. Chromatogr. B, 837 (2006) 29-34.
DOI: 10.1016/j.jchromb.2006.03.063  S. Hann
S. Hann, Z. Stefánka, K. Lenz, G. Stingeder.
Novel separation method for highly sensitive speciation of cancerostatic platinum compounds by HPLC-ICP-MS. Anal. Bioanal. Chem., 381 (2005) 405-412.
DOI: 10.1007/s00216-004-2839-z
 Related EVISA Resources
Related EVISA Resources
 Related EVISA News (Newest first)
Related EVISA News (Newest first)
last time modified: January 11, 2025