Seafood has become very popular in recent time also in countries not having a long coast such as Germany. One of the reasons for its popularity is fast and efficient transportation, allowing the distribution of seafood products even from distant sources, satisfying the consumers with respect to freshness.
The fact that high mercury concentrations in the environment are troublesome is in general known to the public due to alarming news distributed by the press in recent times, as can be seen from some of the headlines of German newspapers. However, that mercury content is a reason to limit the consumption of certain types of seafood is a more recent recommendation (see Focus-headline from 2001) and this is fully understood only by a minority. What is the reason to warn the consumer of the consumption of shark flesh and not of mussels?
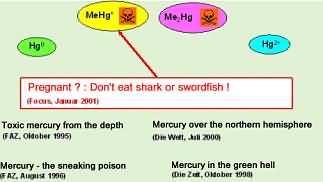
The reason for the headline in Focus in the year 2001 was probably the publication of a study of the U.S. Environmental Protection Agency (EPA) dealing with the impact of methyl mercury on human health. Mercury is present in the environment in different forms such as elemental (metallic) mercury (Hg0), inorganically bound mercury (Hg2+) and organically bound mercury, for example monomethyl mercury (MeHg+) or dimethyl mercury (Me2Hg). These different chemical forms of an element are called “element species”. When assessing the risk for the human health, one has to consider that organically bound mercury species are much more toxic than elemental or inorganic species. The reason for the higher toxicity of organomercury species is the fact that these species can overcome the blood/brain barrier and therefore can damage the central nervous system. Also, the barrier created by the placenta can be overcome, threatening the life of the unborn. The later fact is the reason that
EPA has recommended especially pregnant women to restrict their consumption of contaminated fish.
While elemental mercury can be found in small amounts mainly in the atmosphere, inorganic mercury compounds are present in rock minerals and soil. Dimethyl mercury plays a certain role as an unstable species within the biogeochemical cycling of the element, however has never been found in concentrations threatening human health. Monomethyl mercury however, also called „methyl mercury“ can be bioaccumulated by fish and other aquatic organisms.
The starting point for such bioaccumulation are aquatic microorganisms and algae which are able to metabolise inorganic mercury species into methyl mercury , as has been demonstrated by our research group in some model experiments some years ago. While the mercury concentration in the water most often is extremely low and not threatening, these organic mercury species gets bioaccumulated in fish and other aquatic organisms depending on there position in the food chain.
Mercury concentrations in seawater globally measured by us seldom surpass a level of 0.1 nanogram (ng) per liter of seawater (0.1 ng can also be expressed as 10-10 g or 0.0000000001 gram). However, known concentration levels of mercury compounds in fish are many orders of magnitude higher and can reach the microgram per gram (µg/g) level. The fact that the frequent consumption of fish contaminated by such high mercury concentration can produce life-threatening health problems is known since the 1950’s, when the population of Minamata, a town at the coast of the Japanese Island of Kyushu was poisoned.
Unfortunately, the accumulation of mercury in fish produced by the industrial emission of mercury into the bay of Minamata was unknown at that time and emission control came into action too late. As was found out later, the local population had mainly consumed fish containing methyl mercury at levels of 6-25 µg per gram fish flesh. Little is known about the health effects of much lower concentrations as found naturally in fish. A study with children aged about 7 years from the Färöer-Islands found some developmental deficiencies, which were related to a prenatal exposition to methyl mercury.
We have developed in our laboratory methods for the determination of the extremely low concentrations of methyl mercury in seawater and also for the higher concentrations in seafood. While methods for the determination of total mercury are available since quite some years, the accurate differentiation of the different mercury species was a new challenge.
The detection power necessary for the low concentrations could be achieved by coupling a selective separation technique with a sensitive element –selective detection technique. As can be seen from the photo of our instrumentation, we coupled capillary gas chromatography (CGC) with an inductively coupled plasma-mass spectrometer (ICP-MS) using a thin transfer line.

Figure 3: CGC-ICPMS system
While this CGC-ICPMS hyphenated system has the necessary detection power, still the problem of accurate quantitation remains to be solved as with any other analytical method. Only an appropriate calibration strategy will allow for reliable and accurate quantitation, a requirement especially in food analysis. We have used the principle of isotope dilution analysis, which internationally is known as a reliable and accurate method, due to the limited number of factors influencing the result (no influence of the matrix components, drifting instrumental response, varying yields or possible species transformation during sample pre-treatment). These favourable characteristics are the result of the procedure in which the sample is mixed with a calibration standard consisting of the same element species (methyl mercury) to be analysed, labelled with a stable isotope marker (here with the stable Hg-201 isotope). In contrast to other methods, were the absolute amount of the analyte has to be determined, the isotope dilution method requires only to measure the isotope ratio (here Hg-201/Hg-202) within the species fraction separated by gas chromatography. Since all the mentioned parameters act on both isotopes similarly, the isotope ratio is not influenced at all by these factors. Therefore, the accurate concentration can be obtained from the isotope ratio and the analytical data by relatively simple calculation. The principle of such isotope dilution analysis is schematically shown in the following figure for the mass spectra of the isotopes Hg-201 and Hg-202.
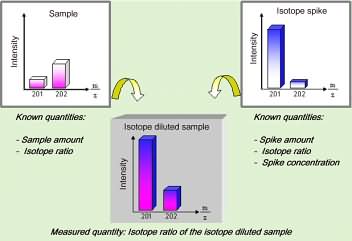
Figure 4: Schematic view of isotopic dilution analysis
Since seafood frequently contains also other organically bound heavy metals, we have developed a CGC-ICPMS method using isotope dilution that is able to determine the three organometallic species in question simultaneously, namely methyl mercury, tributyltin (TBT, Bu3Sn+) and trimethyllead (TML, Me3Pb+). TBT is introduced into the marine environment exclusively by anthropogenic sources such as meanwhile banned antifouling paints preventing mussel and algae growth on ship vessels. TML is a degradation product of the anti-knock agent of leaded gasoline that was in use until recently in Germany but is still in use in other parts of the world. This species can also be created by biological induced methylation of inorganic lead in seawater by algae and microorganisms. A GC chromatogram of a mussel tissue sample, containing all three species and additionally also di- and monobutyltin (DBT, MBT) and the phenyl-compounds is shown in the following figure.
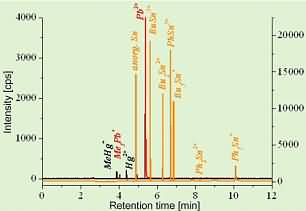
Fig.5: Multi-species chromatogram of a fish sample
The chromatogram has been simplified by indicating only the measured intensities for one of the two isotopes, necessary for isotope dilution analysis within the temporarily separated fractions of the metal species (related to the different retention times within the gas chromatogram). In order to allow the gas chromatographic separation of the non-volatile ionic heavy metal species, the non-volatile species had to be transferred into related volatile species by ethylation.
Meanwhile, we have used this new multi-species method for the analysis of different seafood samples obtained from local seafood shops. While butyltin and methyllead was found only in extremely low concentrations sometimes below the detection limit of 0.3 nanogram per gram for Bu3Sn+ , such was not the case for methyl mercury. Some representative values obtained, meanwhile confirmed by further analysis, are given in the following table. Values given for total mercury and methyl mercury are related to the fresh (wet) sample.
|
Sample |
Concentration of
methyl mercury
(µg/g) |
Concentration of
total mercury
(µg/g) |
Fraction of methyl
mercury in % |
|
shrimps |
0.007 |
0.009 |
78 |
|
mussels |
0.015 |
0.032 |
47 |
|
plaice |
0.028 |
0.030 |
93 |
|
coalfish |
0.140 |
0.156 |
90 |
|
tuna |
0.265 |
0.290 |
91 |
|
shark |
2.79 |
2.85 |
98 |
What can be concluded from the analysis of seafood samples been done so far ?
-
A high percentage of the total mercury in fish and other seafood samples is present in the form of the extremely toxic methyl mercury species. Obviously, the fraction of methyl mercury but also its absolute amount is growing with the age of the marine organism. This seems reasonable, since it is well known that inorganic mercury species can be excreted by organisms much faster than organic mercury species. Further, those carnivore fishes such as the shark, being situated at the end of the marine food-chain, accumulate methyl mercury over a long period. The methyl mercury fraction measured by us in shark samples was always in the range of 80-100% with concentration levels most often exceeding 1 µg/g.
-
While a frequent consumption of shrimps and mussels seems to be safe with respect to methyl mercury, consumption of shark meat should be restricted. Following the recommendation given by EPA (intake of 0.1 µg methyl mercury per kg body weight and day), a person weighing 70 kg could reach this safety level already by consuming only 2.5 gram of shark meat. But even when using less restrictive recommendations such as that one by the Australian Dietary Guidelines, recommending restricting the intake of methyl mercury to a maximum of 3.3 µg methyl mercury per kg body weight and week, the safety limit for the weekly intake could be reached by consuming only 80 grams of shark meat.
What are the necessary actions related to this analytical perspective ?
-
Legislation should recognize that concentration levels of total mercury are not meaningful for limiting mercury contamination in foodstuffs, since only methyl mercury levels are adequate for describing the risk for human health.
-
Governmental food control laboratories should be empowered by appropriate instrumental equipment and know-how to determine the different mercury species and to quantitate them reliably.
-
A routine analytical method based on the current state-of-the art should be used for the reliable determination of methyl mercury. Since isotopic-labelled methyl mercury is today also commercial available, such method could be based on species-specific isotope dilution analysis using a CGC-ICPMS system as a reliable and very sensitive technique.
-
The know-how of speciation analysis, which at this moment has been collected and developed mainly at universities, should be promoted and transferred in order to improve the situation in governmental institutions and other more routinely working laboratories. Due to the improved information value provided by elemental speciation analysis this analytical methodology plays an important role not only in the case of methyl mercury but many more cases. To name some examples highlighting the importance of speciation analysis we would like to mention arsenic, were speciation analysis allows for the differentiation of the more toxic inorganic species and the less toxic organic species such as arsenobetaine or arsenocholine, or chromium, were speciation analysis can differentiate between the hexavalent chromium that is classified as carcinogen and the trivalent chromium that is recognized as being essential.
-
The collection of information and know-how, its dissemination and transfer towards all those who seek for species related information and the improvement of education with respect to elemental speciation analysis are some of the main activities of the Virtual Institute for Speciation Analysis (EVISA). EVISA provides training courses for techniques and methods as those mentioned above (GC-ICP-MS, species-specific isotope dilution analysis). For a complete list of courses please consult our list under the section training.
 Related Studies
Related Studies

Nataliya Poperechna,
Klaus Gustav Heumann,
Simultaneous multi-species determination of trimethyllead, monomethylmercury and three butyltin compounds by species-specific isotope dilution GC-ICP-MS in biological samples, Anal. Bioanal. Chem., 383/2 (2005) 153-159.
DOI: 10.1007/s00216-005-0036-3
Nataliya Poperechna,
Klaus G. Heumann,
Species-Specific GC/ICP-IDMS for Trimethyllead Determinations in Biological and Environmental Samples, Anal. Chem., 77/2 (2005) 511-516.
DOI: 10.1021/ac048757m

Lorenzo Perna, Angela LaCroix-Fralish,
Stefan Stürup,
Determination of inorganic mercury and methylmercury in zooplankton and fish samples by speciated isotopic dilution GC-ICP-MS after alkaline digestion, J. Anal. At. Spectrom., 20/3 (2005) 236-238.
DOI: 10.1039/b410545a James P. Snell
James P. Snell, Christophe R. Quétel, Lars Lambertsson, Johanna Qvarnström,
Preparation and certification of ERM-AE670, a 202Hg enriched methylmercury isotopic reference material, J. Anal. At. Spectrom., 19/10 (2004) 1315-1324.
DOI: 10.1039/b407368a

R.C. Rodríguez Martín-Doimeadios,
Eva Krupp,
David Amouroux,
Olivier F.X. Donard,
Application of Isotopically Labeled Methylmercury for Isotope Dilution Analysis of Biological Samples Using Gas Chromatography/ICPMS, Anal. Chem., 74/11 (2002) 2505-2512.
DOI: 10.1021/ac011157s

Natascha Demuth,
Klaus G. Heumann,
Validation of Methylmercury Determinations in Aquatic Systems by Alkyl Derivatization Methods for GC Analysis Using ICP-IDMS, Anal. Chem., 73/16 (2001) 4020-4027.
DOI: 10.1021/ac010366+ James P. Snell
James P. Snell, Ian I. Stewart,
Ralph E. Sturgeon,
Wolfgang Frech,
Species specific isotope dilution calibration for determination of mercury species by gas chromatography coupled to inductively coupled plasma- or furnace atomisation plasma ionisation-mass spectrometry, J. Anal. At. Spectrom., 15/12 (2000) 1540-1545.
DOI: 10.1039/b007130g
 Related Information
Related Information
 FDA: Mercury Levels in Commercial Fish and Shellfish
FDA: Mercury Levels in Commercial Fish and Shellfish
 Related EVISA News (newest first)
Related EVISA News (newest first)
 January 21, 2011: Arctic Mercury Cycling May Be Linked to Ice Cover
January 21, 2011: Arctic Mercury Cycling May Be Linked to Ice Cover June 28, 2010: New Study Examines Why Mercury is More Dangerous in Oceans
June 28, 2010: New Study Examines Why Mercury is More Dangerous in Oceans September 8, 2009: Inorganic Mercury Level in US Women increases
September 8, 2009: Inorganic Mercury Level in US Women increases August 21, 2009: USGS Study Reveals Mercury Contamination in Fish Nationwide
August 21, 2009: USGS Study Reveals Mercury Contamination in Fish Nationwide May 3, 2009: Ocean mercury on the rise
May 3, 2009: Ocean mercury on the rise February 11, 2009: Mercury in Fish is a Global Health Concern
February 11, 2009: Mercury in Fish is a Global Health Concern March 11, 2007: Methylmercury contamination of fish warrants worldwide public warning
March 11, 2007: Methylmercury contamination of fish warrants worldwide public warning October 9, 2006: Linking atmospheric mercury to methylmercury in fish
October 9, 2006: Linking atmospheric mercury to methylmercury in fish February 9, 2006: Study show high levels of mercury in women related to fish consumption
February 9, 2006: Study show high levels of mercury in women related to fish consumption January 12, 2005: Number of fish meals is a good predictor for
the mercury found in hair of environmental journalists
January 12, 2005: Number of fish meals is a good predictor for
the mercury found in hair of environmental journalists April 27, 2004: FDA/EPA recommends pregnant women to restrict
their fish consumption because of methylmercury content
April 27, 2004: FDA/EPA recommends pregnant women to restrict
their fish consumption because of methylmercury content
last time modified: October 15, 2024